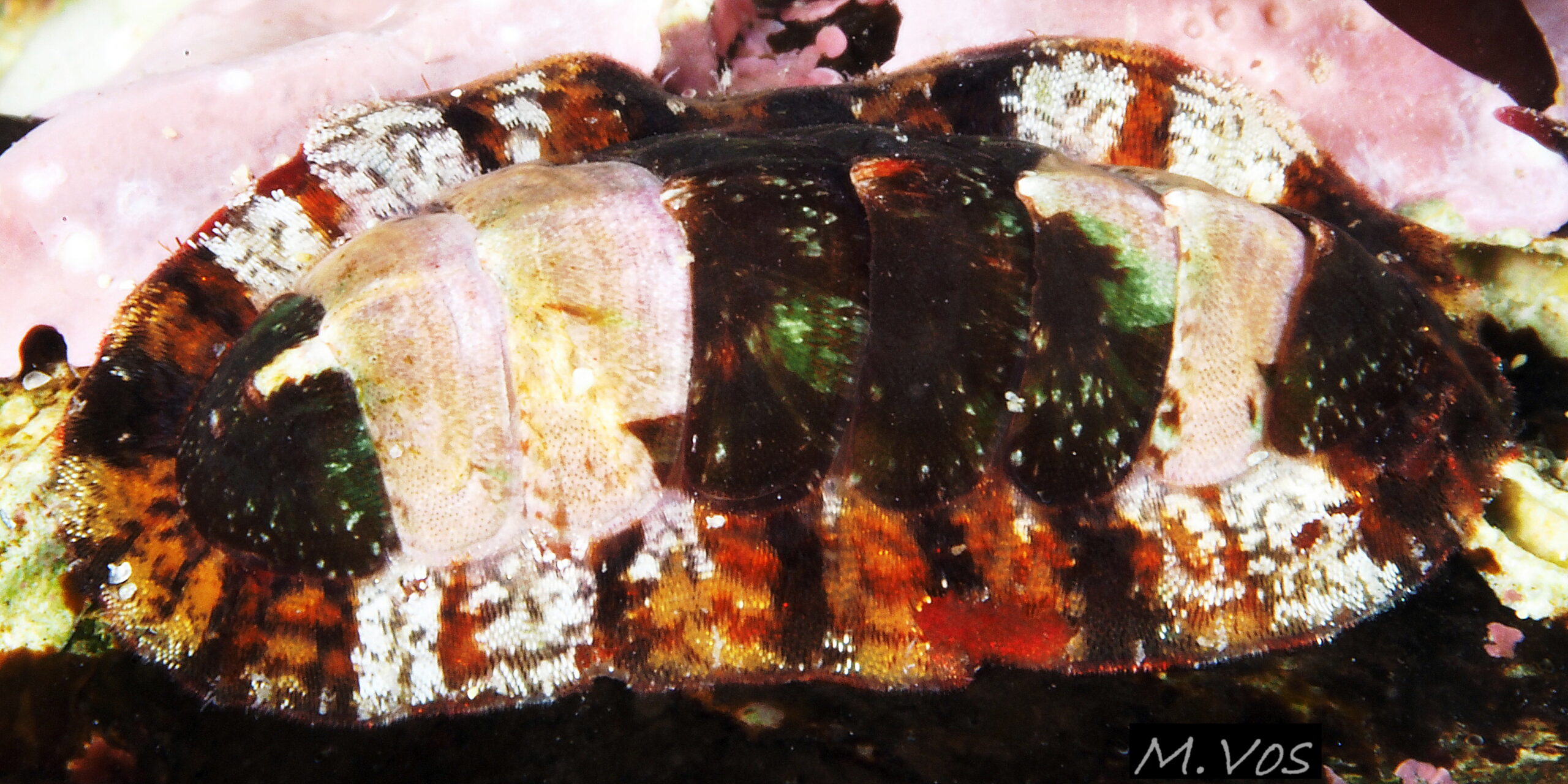Click image to enlarge with full caption. Main text below slider.
Callochiton septemvalvis (Montagu, 1803)
Synonyms: Chiton septemvalvis Montagu, 1803; Callochiton achatinus (T. Brown, 1823);
Chiton laevis Pennant, 1777 sensu Montagu, 1803; Chiton laevis var. navicula Jeffreys, 1865.
Current Taxonomy: WoRMS https://www.marinespecies.org/aphia.php?p=taxdetails&id=140132
Meaning of name; septemvalvis (Latin) = seven valved.
Original description by Montagu (1803) of a specimen with one intermediate valve missing;
“C. with seven carinated valves, strongly beaked, the five middle ones divided transversely from the anterior base to the beak, the hinder compartment very fine shagreen, the other very smooth, or faintly striated transversely: the extreme valve at each end rufous brown, the rest generally dark cinereous; beaks frequently rufous; margin moderately broad, and faintly reticulated: – – length half an inch.”
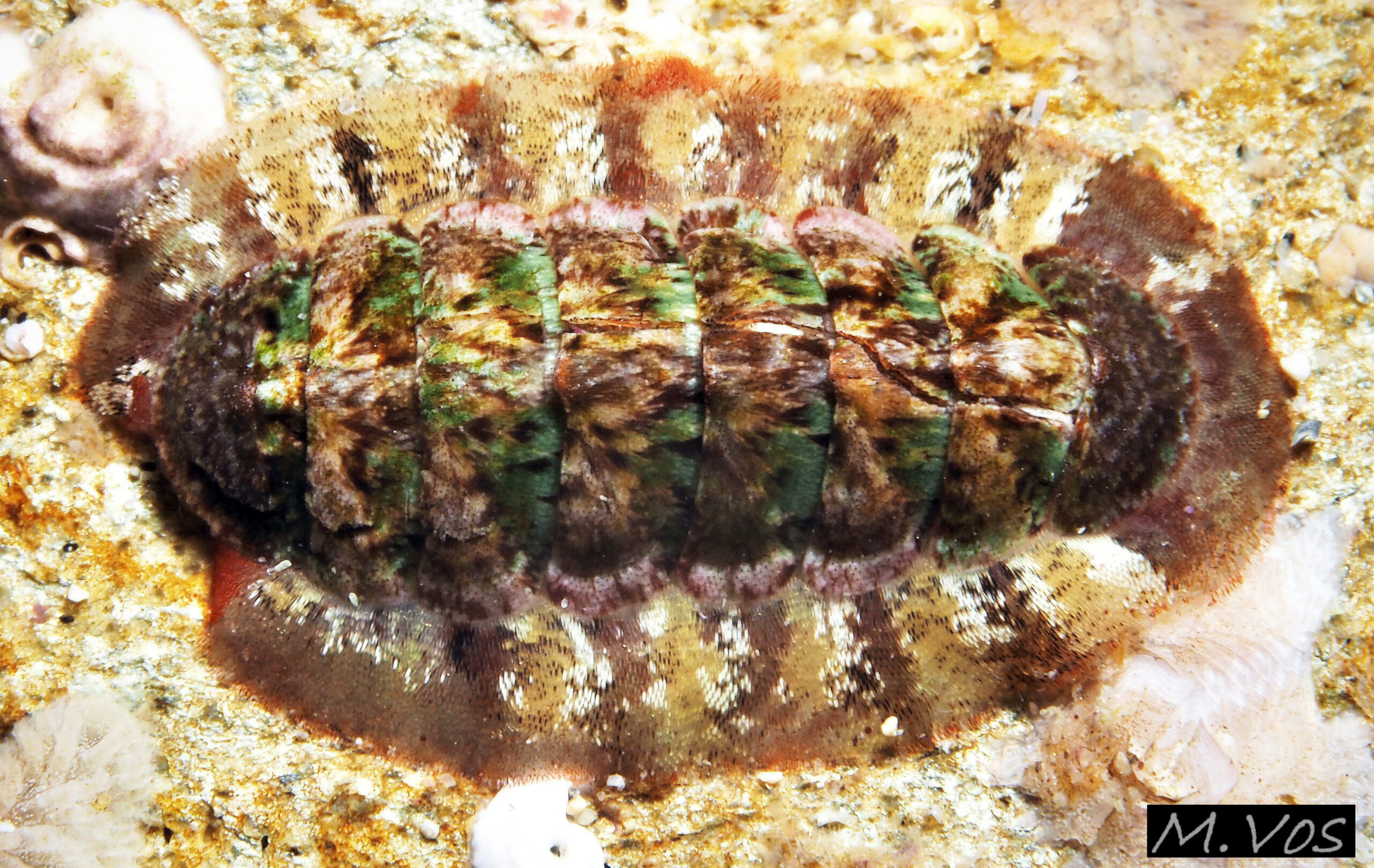
Example of similarly deformed six valved Lepidochitona cinerea in 01 C. septemvalvis .
GLOSSARY BELOW
Shell Description
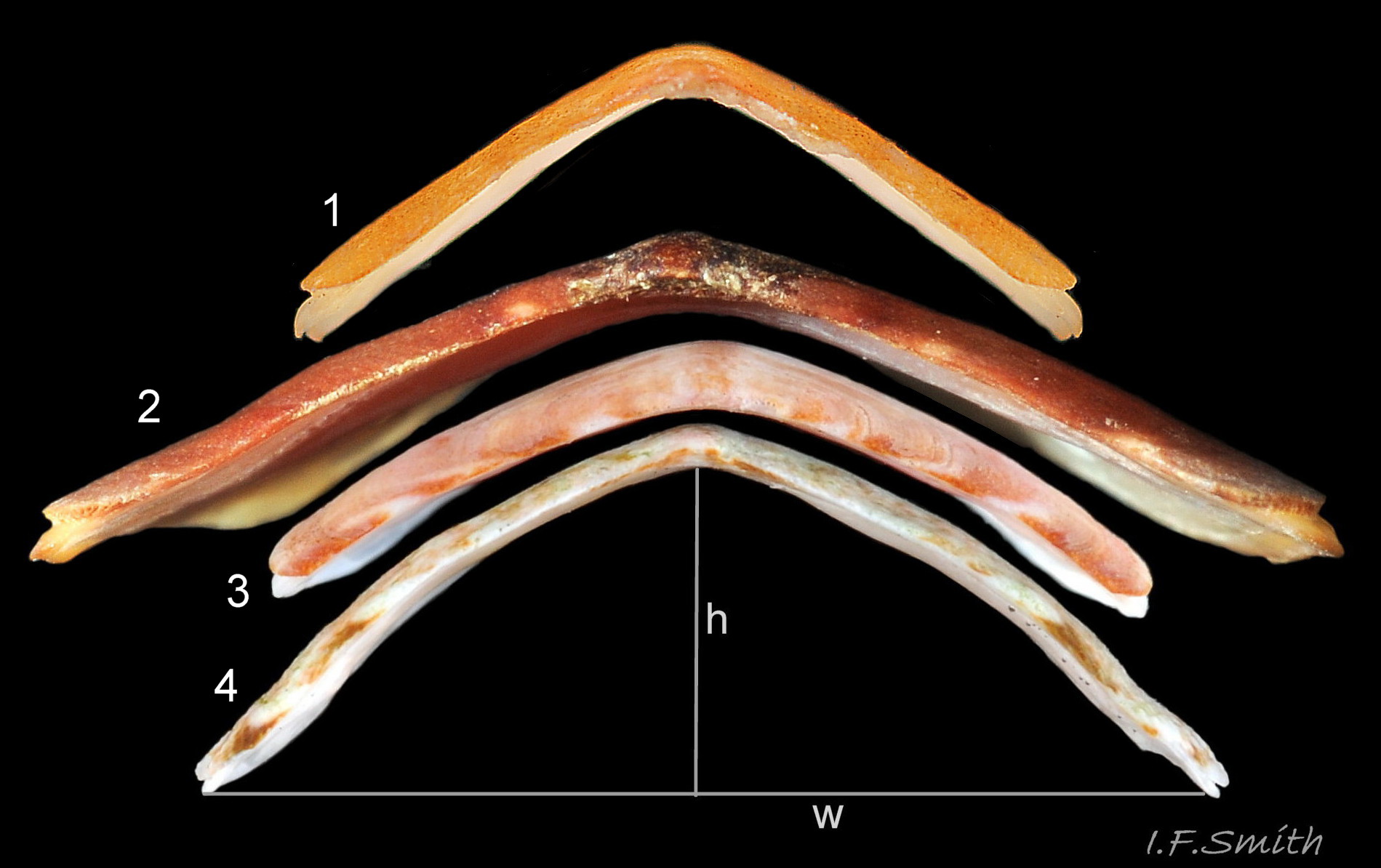
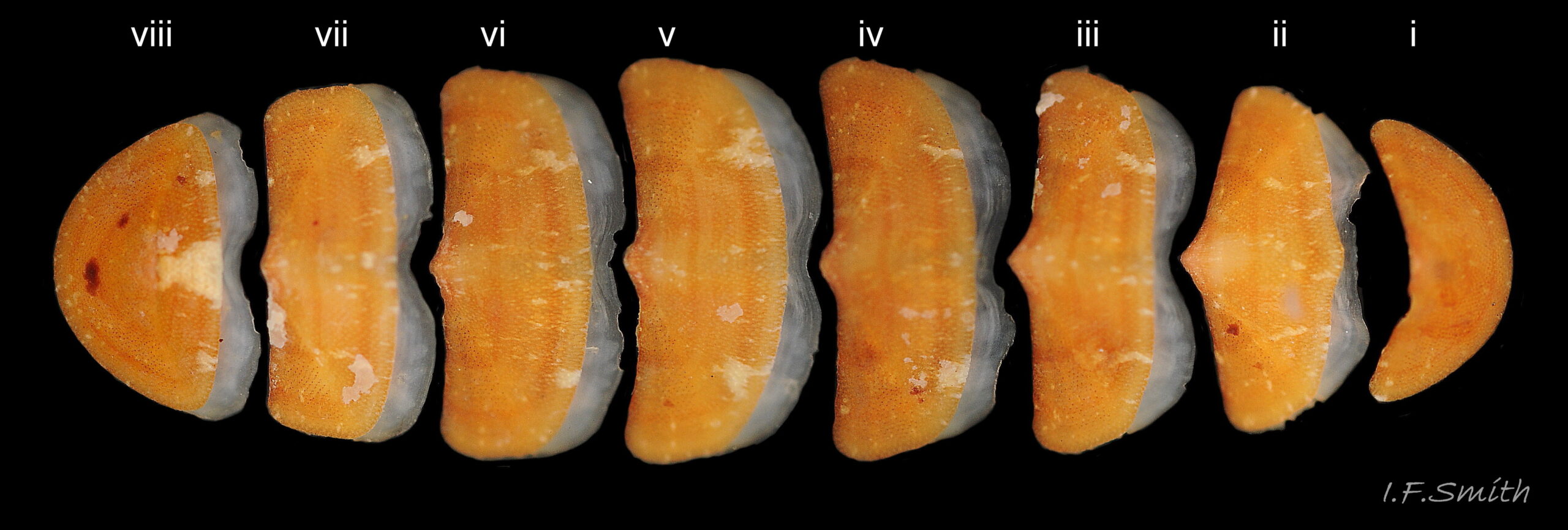
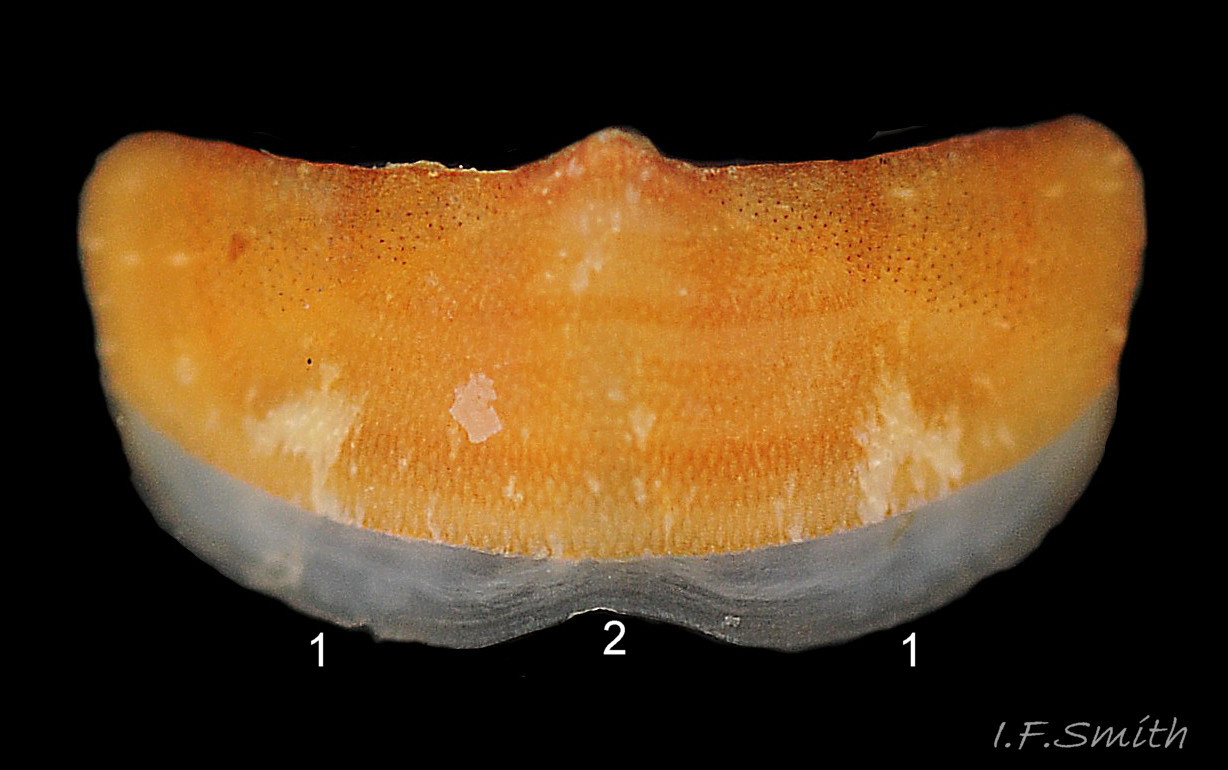
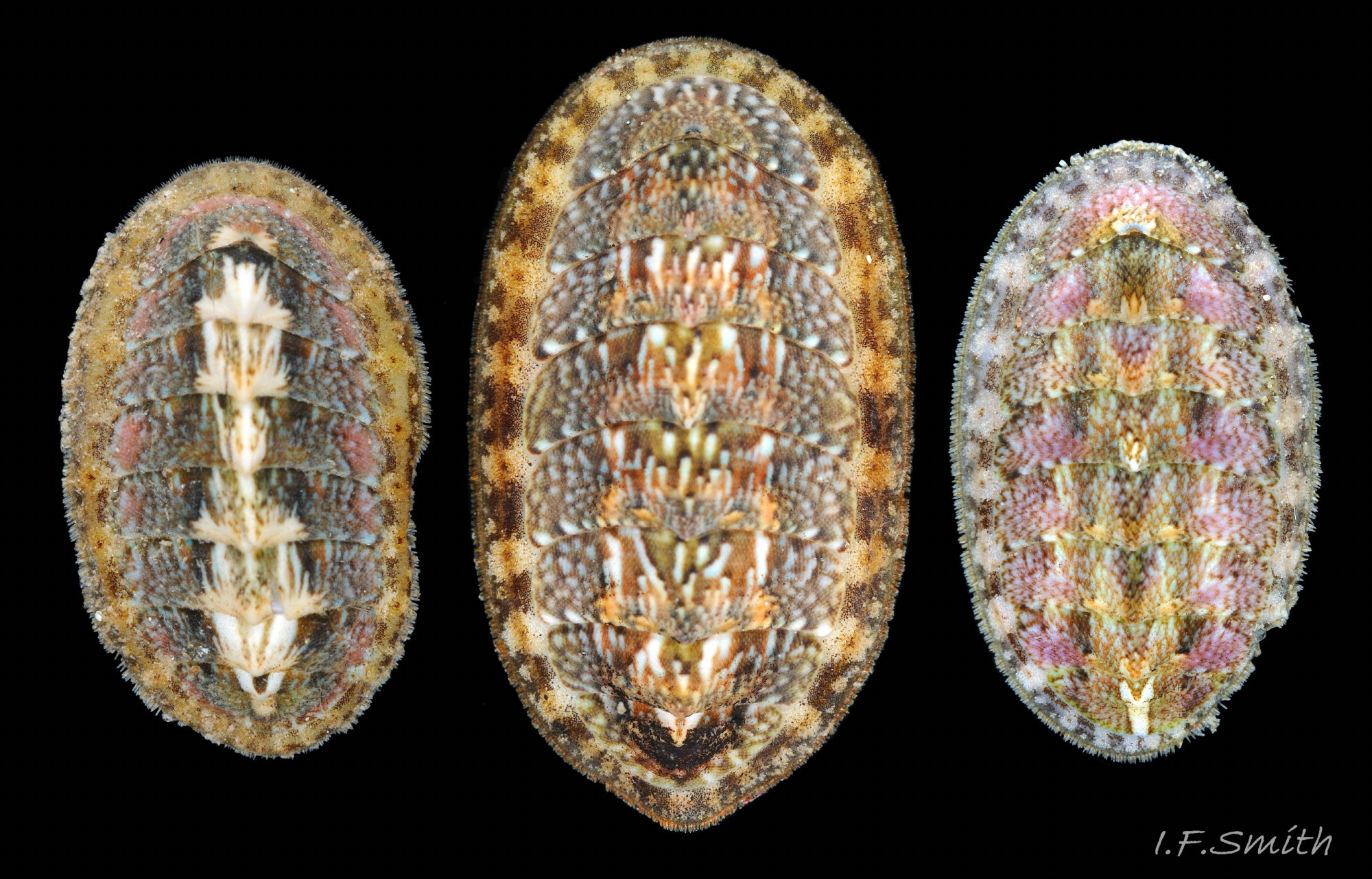
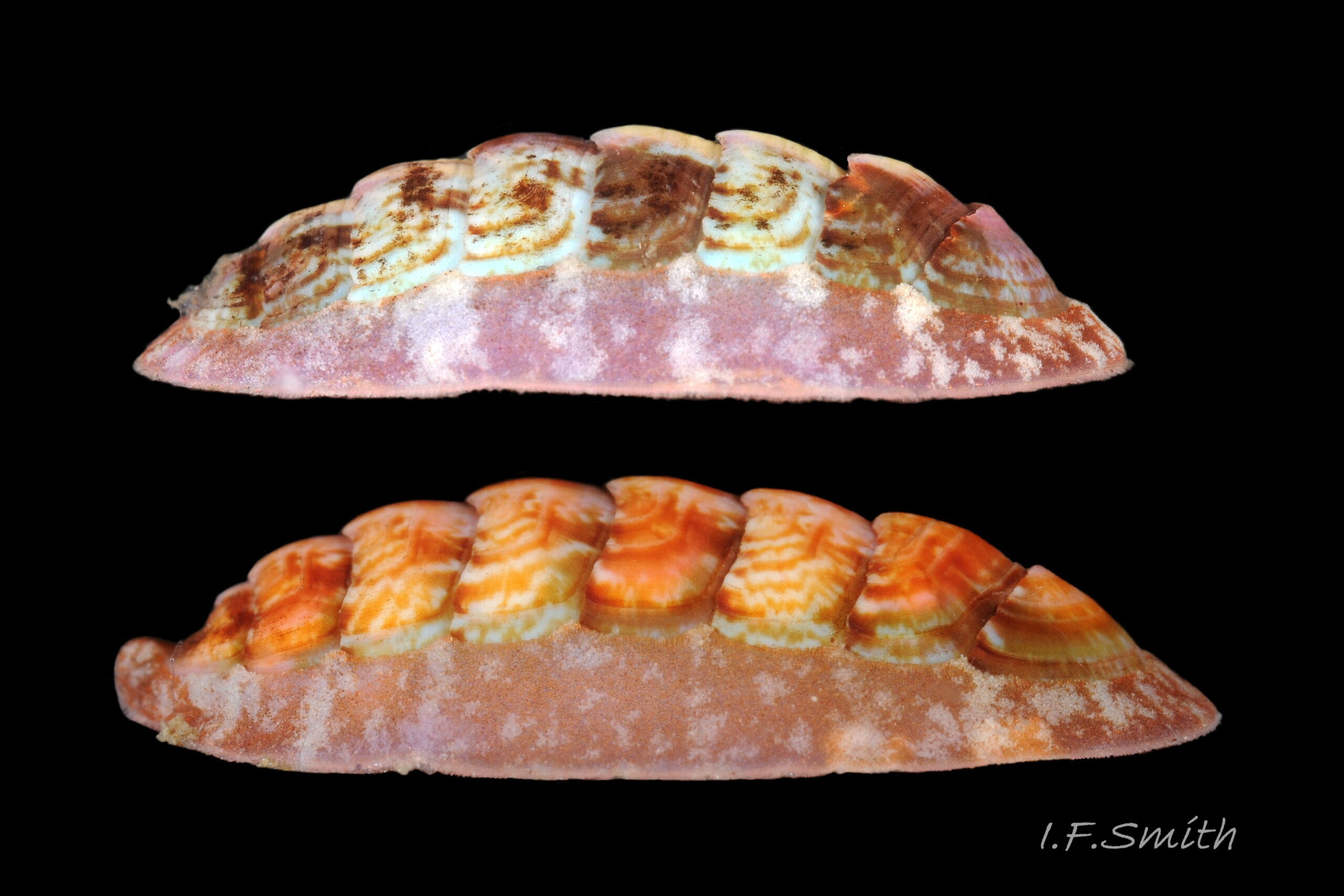
C. septemvalvis is usually up to 22 mm long (Kaas & Van Belle, 2003), exceptionally 32 mm (Jones & Baxter, 1987). In dorsal view, its outline is oval with variable width up to about 60% of its length 02 C. septemvalvis . It is distinctly keeled 03 C. septemvalvis with a pronounced posterior beak 04 C. septemvalvis on each intermediate valve which usually erodes and becomes less prominent with age (Jeffreys, 1865).
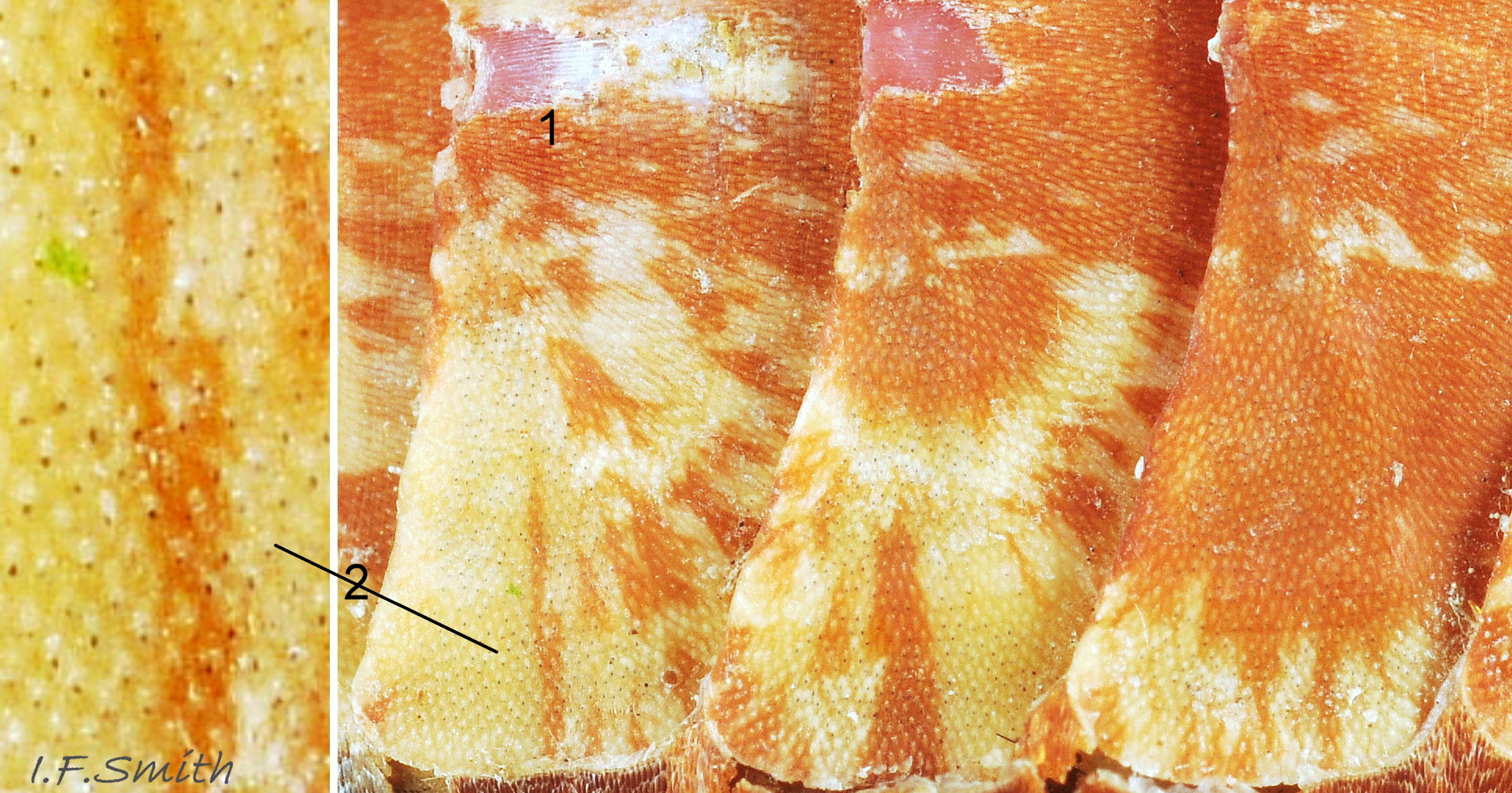
The side slopes of valves ii to vii are virtually flat and their elevation is about 35% to 40% of their width 05 C. septemvalvis . Higher elevations in Kaas & Van Belle (2003) refer to Mediterranean specimens currently accepted as Callochiton doriae (Capellini, 1859). The dorsal surface varies from bright red 06 C. septemvalvis or cerise 07 C. septemvalvis to dark brown 02 C. septemvalvis , often variegated 08 C. septemvalvis with browns, cream, green, bright yellow and/or orange. Some are almost completely brown or cream. The head and tail valves are often distinctly darker 09 C. septemvalvis & 10 C. septemvalvis than the intermediate valves.
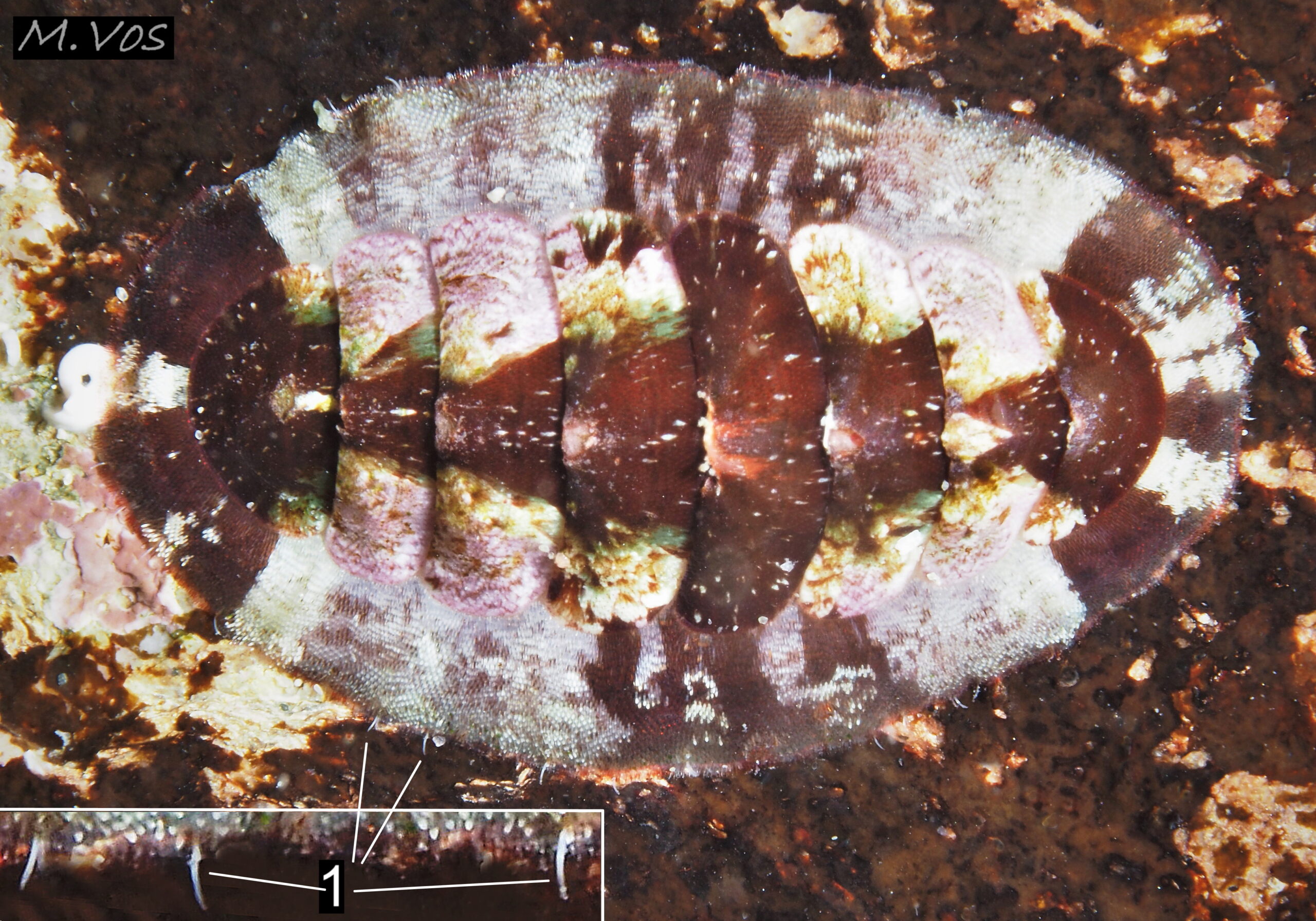
When the girdle is fully spread, it is up to about 40% the width on each side of exposed valve v 08 C. septemvalvis , 09 C. septemvalvis & 11 C. septemvalvis . It has similar colours to the valves and there is frequently an obliquely transverse band of cream on either side by the suture between valves i and ii and valves vii and viii 12 C. septemvalvis . Sometimes there are more bands 07 C. septemvalvis , with up to seven on each side 08 C. septemvalvis .
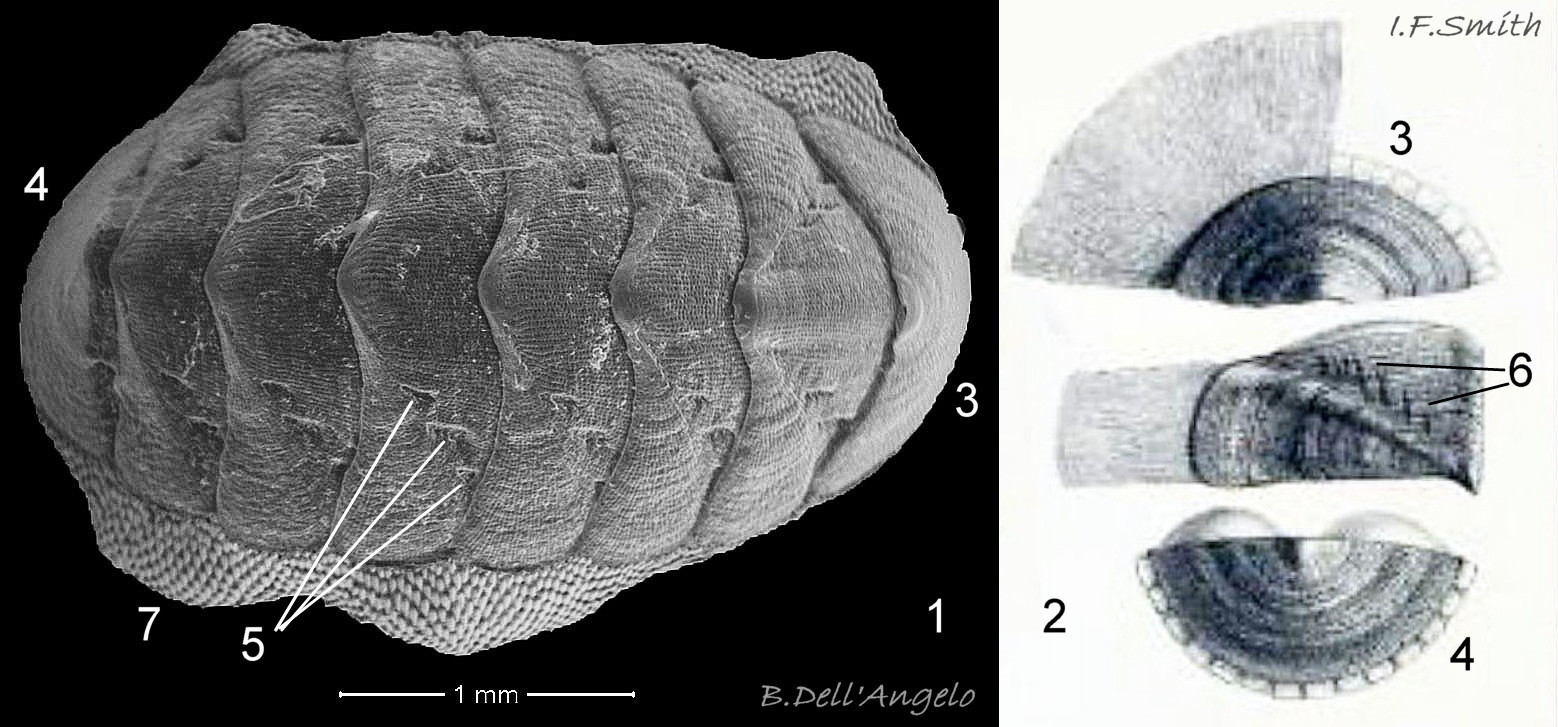
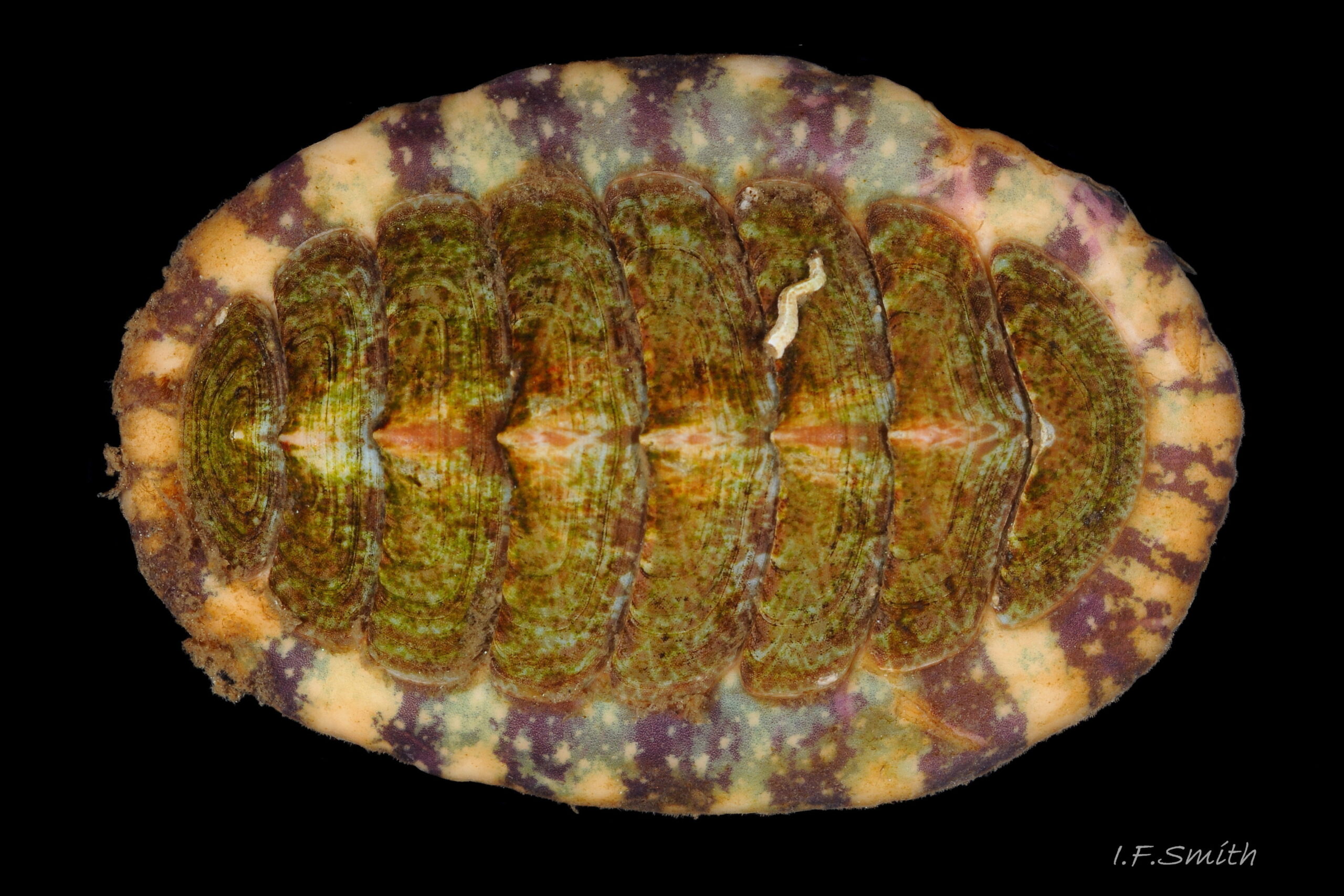
Like other chitons, a normal specimen has eight valves which are conventionally numbered i to viii from head to tail 13 C. septemvalvis . The valves consist of a dorsal ‘tegmentum’ layer of aragonite permeated by canals containing sensory matter called aesthetes, and an inner, translucent ’articulamentum’ layer showing the pinkish underside of the tegmentum 14 C. septemvalvis , 15 C. septemvalvis & 16 C. septemvalvis .
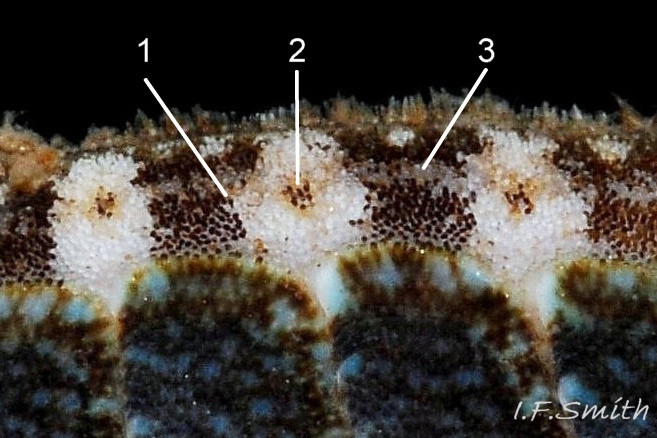
The aesthetes open on the tegmentum surface as small pores arranged in a pattern of quincunxes 16 C. septemvalvis . The smooth valve surface appears to be slightly granular when seen under a hand lens (Jones & Baxter, 1987) 07 C. septemvalvis & 12 C. septemvalvis . Many of the aesthete pores in anterior valve i, the post mucronal area of posterior valve viii, and the lateral area of valves ii to vii, contain small black spots 04 C. septemvalvis & 16 C. septemvalvis .
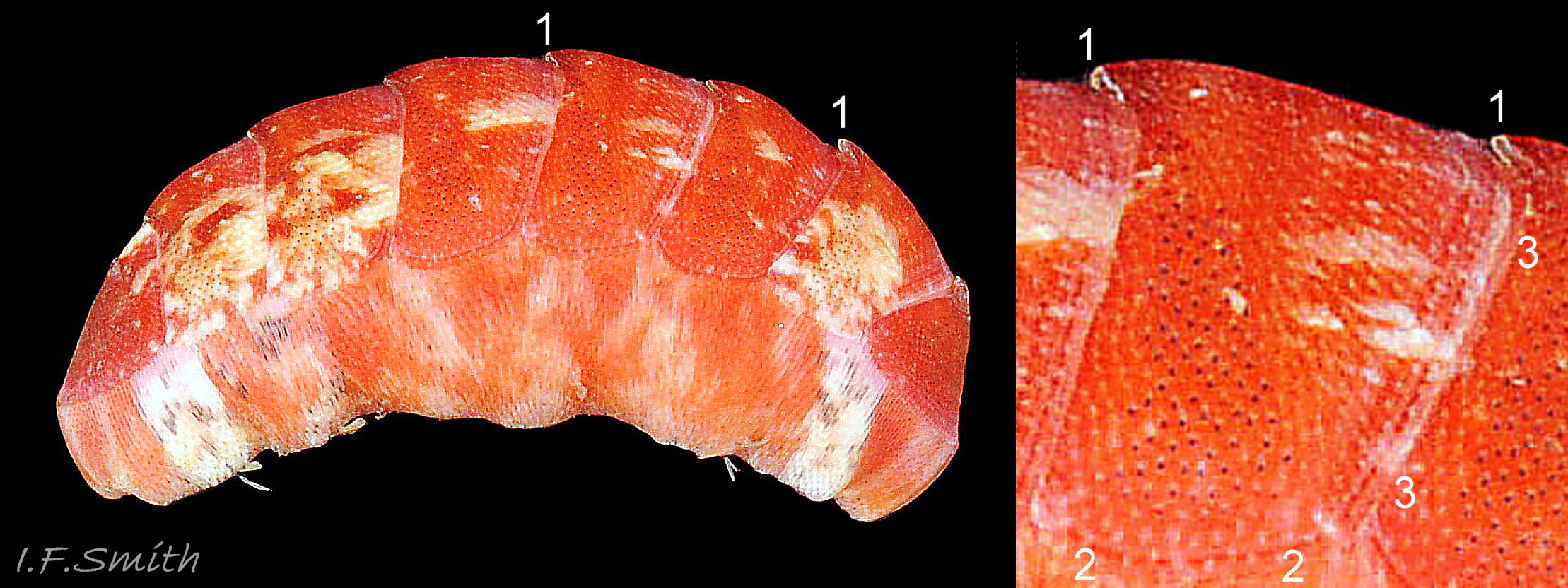
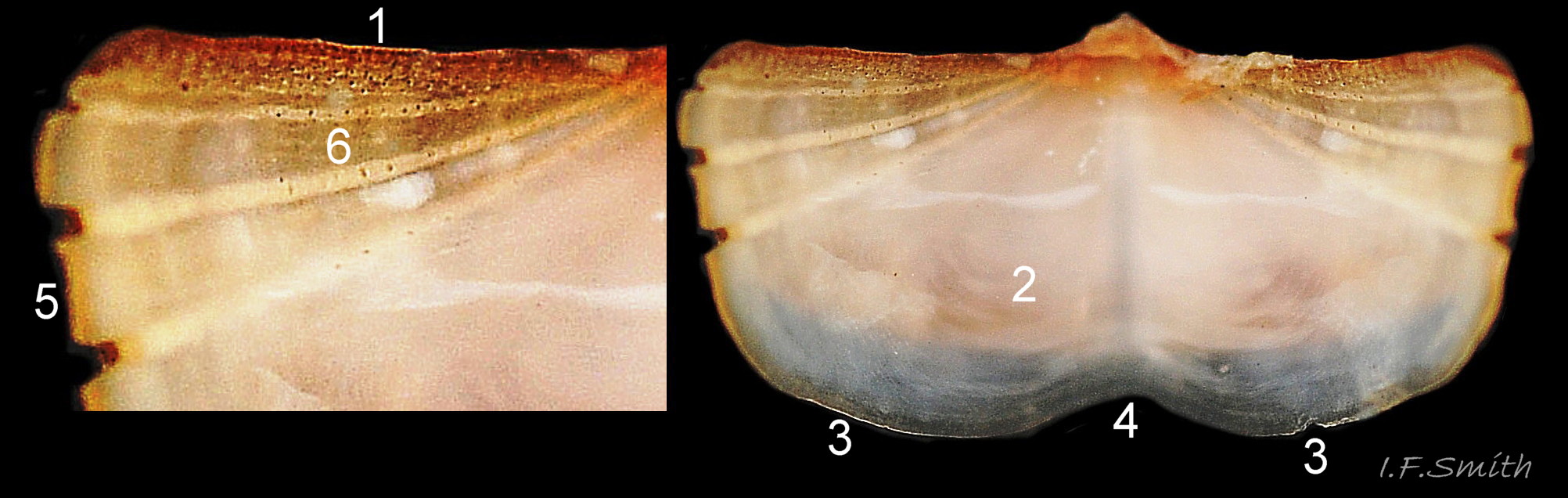
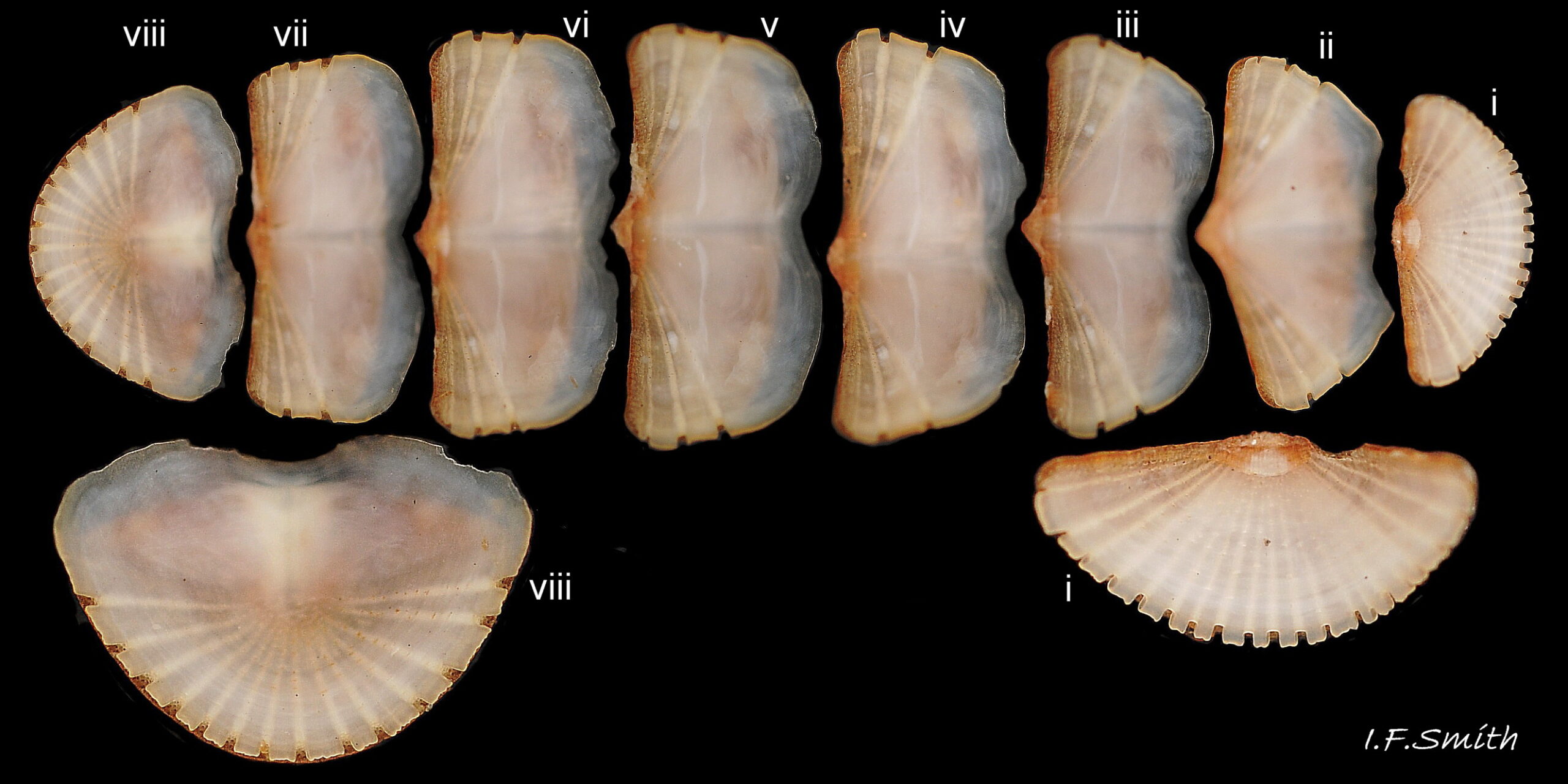
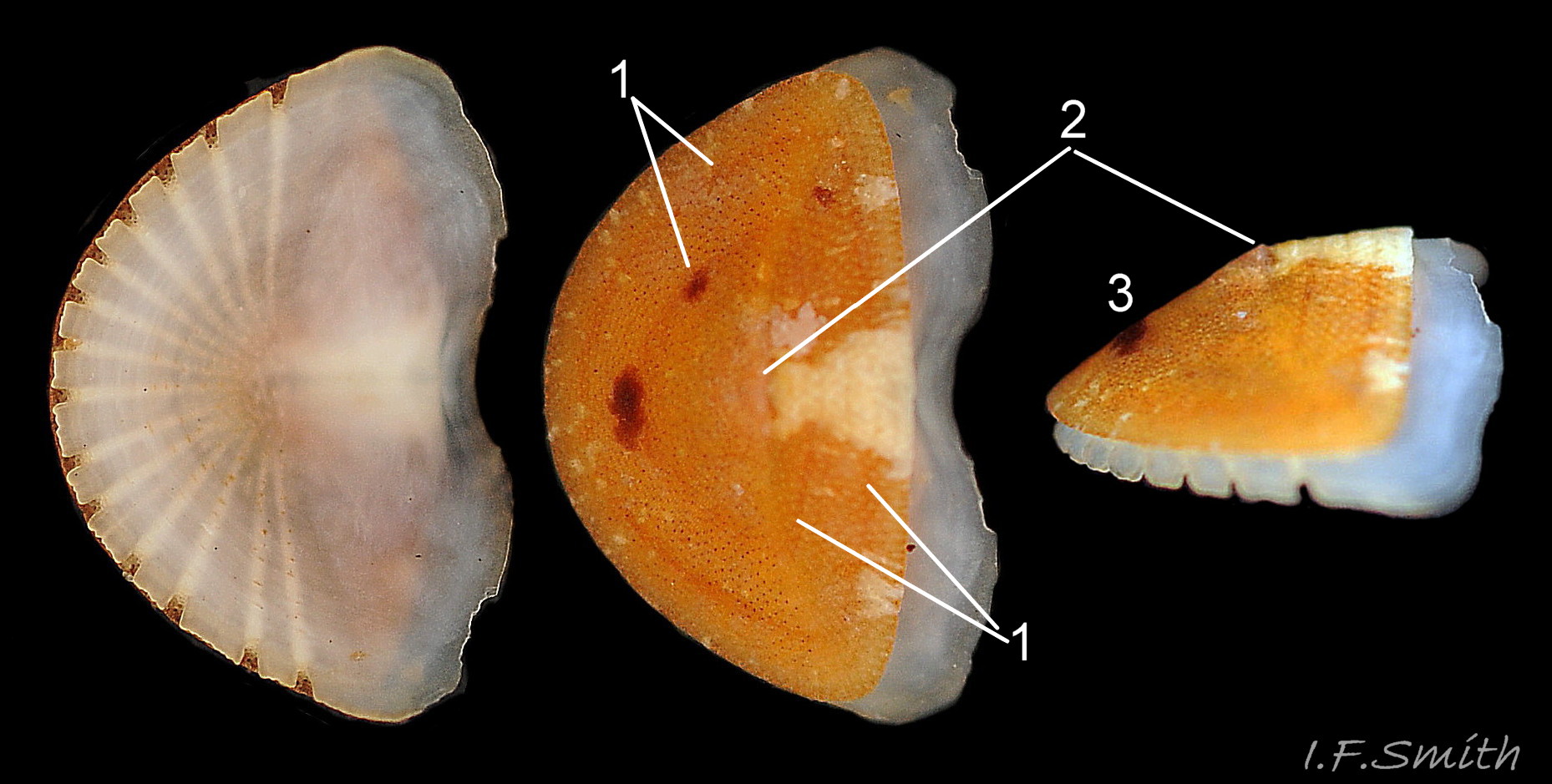
Each side of the intermediate valves, ii to vii has a triangular lateral area from the beak to the lateral edge of the valve, and a triangular pleural area from the beak to the anterior edge of the valve 04 C. septemvalvis . The boundary between the two areas is marked on some by an abrupt elevation of surface at the commencement of the lateral area 07 C. septemvalvis which is often raised slightly in general, but this is not discernible in some photographs 02 C. septemvalvis . The articulamentum of valves ii to vii protrudes at the anterior as two large, translucent white apophyses which have only a narrow, very shallow jugal sinus between them so they appear to unite 17 C. septemvalvis . On a live individual, the apophyses are attached by strong muscles to the underside of the adjacent valve. On valves ii to vii, the articulatum also extends laterally as short insertion plates cut on each side by slits into two or three rectangular teeth which insert into the girdle 14 C. septemvalvis . Other chiton species in north-west Europe have a single slit per side of valves ii to vii. On the surface of the articulamentum there are pale rays which have pores where the aesthetes connect the valves to the mantle 14 C. septemvalvis .
The head valve (i) is semicircular with the articulatum extending all round the curved anterior-lateral edge where 16 to 20 slits cut it into rectangular teeth, which insert into the surrounding girdle 18 C. septemvalvis . In dorsal view with the curved edge on the substrate, the posterior margin of valve i is widely V-shaped. In lateral view, valve i has a flat to slightly concave anterior slope. The ventral surface has a pale slit-ray with round pores leading to each of the 16 to 20 slits 18 C. septemvalvis .
In dorsal view, the posterior edges of the intermediate valves (ii –vii) vary between gently concave, almost straight and slightly convex, with a medial, triangular apex/beak when uneroded 13 C. septemvalvis . The anterior edge of the tegmentum is gently convex and the more convex apophyses, which are conjoined at a shallow jugal sinus, project beyond it 17 C. septemvalvis .
The tail valve (viii) is semicircular with semicircular growth lines and a small, unobtrusive central mucro from which the flat posterior slope descends at an angle 19 C. septemvalvis . Its articulamentum extends all round the curved posterior-lateral edge where 14 to 16 slits cut it into rectangular teeth. The articulamentum has a pale slit-ray with round pores leading to each of the slits 19 C. septemvalvis .
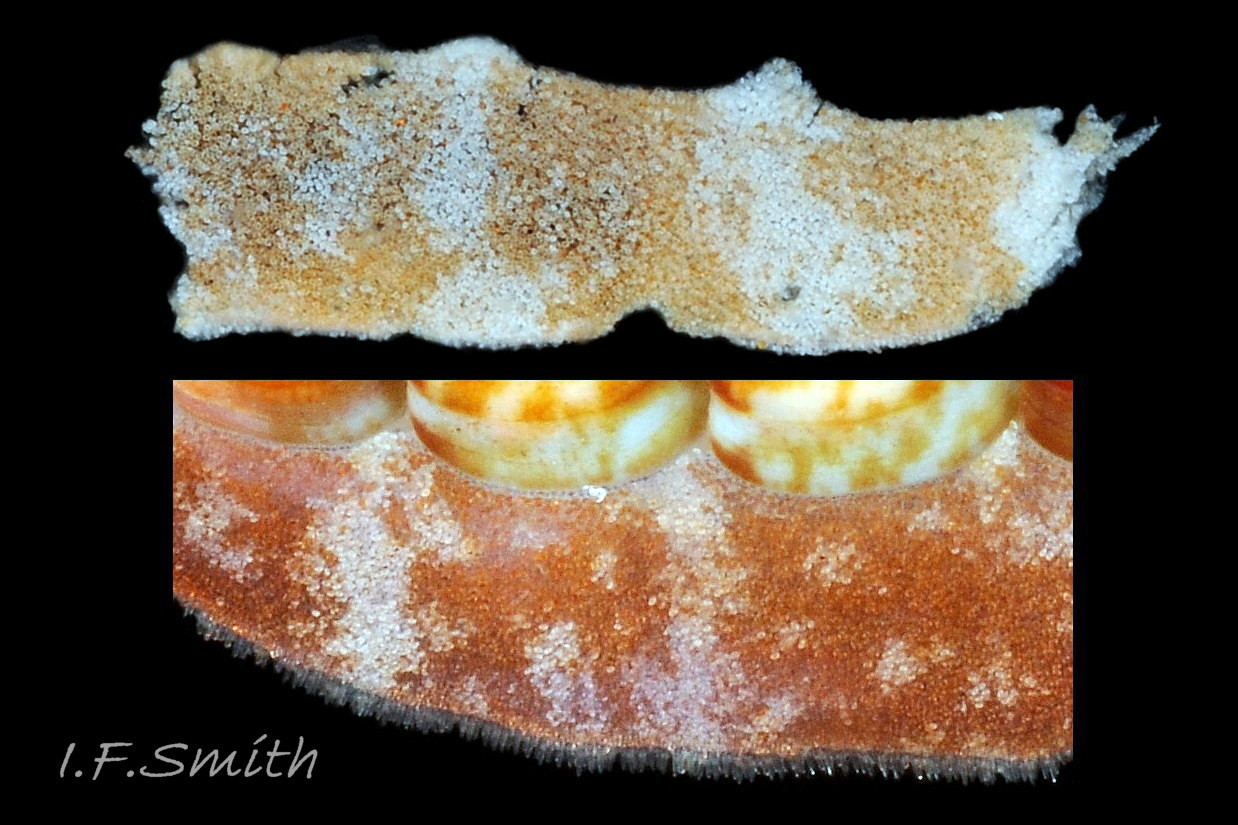
The girdle is entirely covered with transversely arranged, straight, smooth, flattened, imbricated, c. 0.15 to 0.28 mm long needles which are closely appressed to the surface. They are most easily discerned when of a variety of colours 08 C. septemvalvis & 09 C. septemvalvis but are often indistinct when the girdle is uniformly dark 02 C. septemvalvis . Larger, up to 0.6 mm long, slender, curved, white spines are arranged sparsely around, and protrude from, the edge of the girdle 20 C. septemvalvis . The ventral face of the girdle is covered with transverse rows of small, bluntly pointed, flat spicules or scales, up to 0.05 mm long (Kaas & Van Belle, 2003).
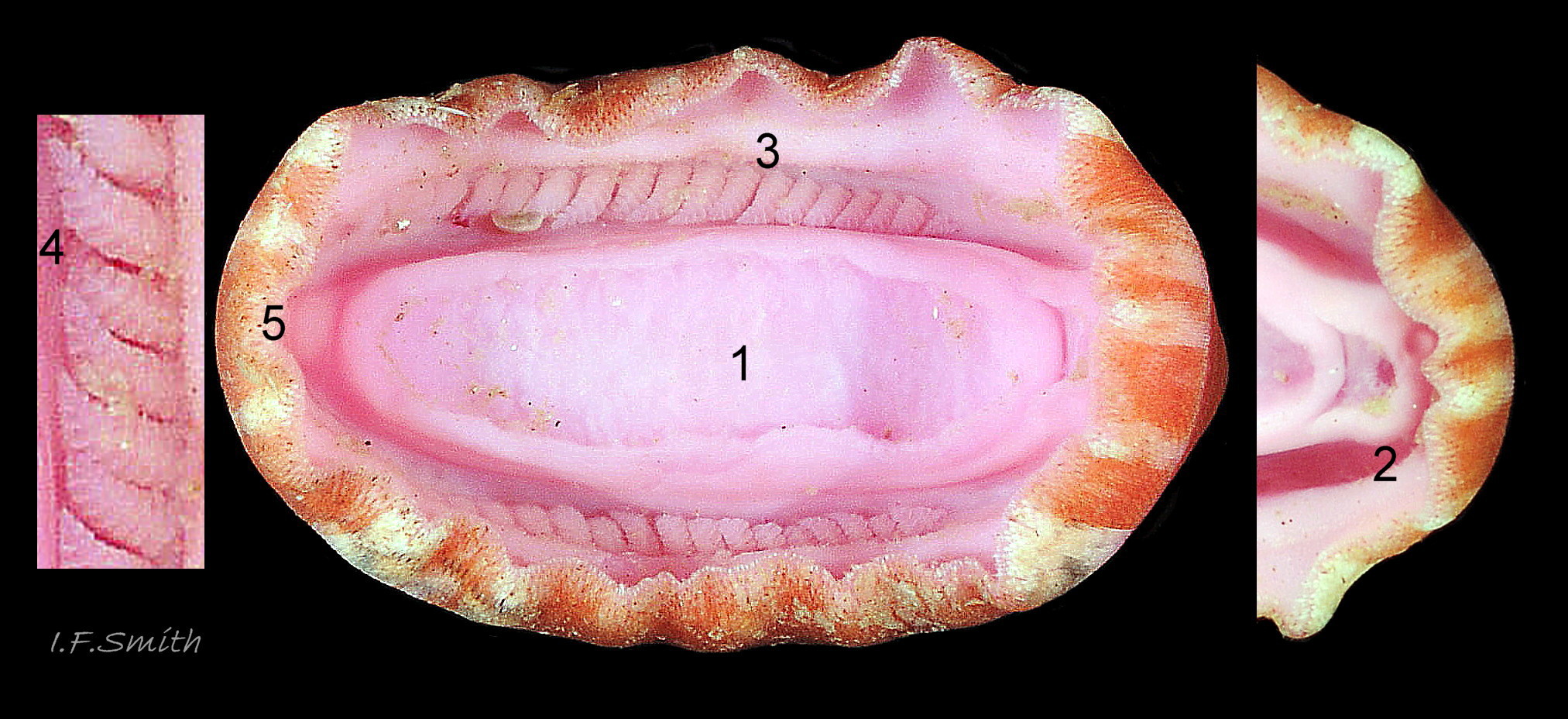
Body Description 21 C. septemvalvis .
The head and foot rarely protrude into view naturally on live individuals, and best seen if the chiton is removed from the substrate, or attached to glass.
The head consists of a mouth surrounded by the anterior of the foot and a large antero-lateral rim which extends laterally onto the sides of the foot; it has no eyes or sensory tentacles.
An open, narrow mantle-cavity runs around the whole animal. On each side it contains about 20 simply pinnate, fleshy gills. They extend nearly the whole length of the foot (holobranch arrangement). The anus, on a large papilla, opens into the mantle-cavity at the posterior. Excretory nephridiopores and gonopores open laterally into the posterior quarter of the cavity. There is no penis as fertilization is external.
Key identification features
Callochiton septemvalvis
1) Maximum length 32 mm. Intermediate valves H/W c.35% to 40% 02 C. septemvalvis .
2) Valves ii to vii have straight, or nearly straight, side slopes 05 C. septemvalvis and no longitudinal grooves in the pleural areas.
3) Dorsal surface of valves varies bright red 06 C. septemvalvis to dark brown 02 C. septemvalvis variegated with other colours 08 C. septemvalvis . Head and tail valves, i and viii, often darker 09 C. septemvalvis than intermediate valves.
4) Girdle often with a cream band by sutures i/ii and vii/viii and frequently by other sutures 02 C. septemvalvis .
5) Dorsal surface of girdle has densely packed, transversely arranged, 0.15 to 0.28 mm long needles which are closely appressed to the surface (Kaas & Van Belle, 2003) 08 C. septemvalvis & 09 C. septemvalvis .
6) Minute black aesthete spots on lateral areas of intermediate valves 04 C. septemvalvis & 16 C. septemvalvis ; no other chiton sp. in north-east Atlantic has this feature documented. Dorsal surface of valves slightly granular when seen under a hand lens (Jones & Baxter, 1987). Magnification and good lighting of clean specimens are usually needed to discern the spots and granulation.
7) Only British species with two or three slits and slit rays in the articulamentum on either side of the intermediate valves, ii to vii.
Apophyses on valves have only a narrow, very shallow jugal sinus between them 17 C. septemvalvis .
8) Usually 20 to 25 gills each side, arrangement holobranch 21 C. septemvalvis .
10) Atlantic from north Norway to Iberia and the Canary Islands; absent from inner Baltic. South, west and north coasts of Britain, rarely numerous. Scarce or absent in southern North Sea and north-east Irish Sea.
11) Mucro near centre of tail valve viii 19 C. septemvalvis .
Similar species
Callochiton doriae (Capellini, 1859)
Regarded as a Mediterranean form of C. septemvalvis by Kaas & Van Belle (2003) and Jones & Baxter (1987) but currently (2023) accepted as a separate species on WoRMS, following molecular sequencing (Sigwart et al., 2013 cited in Dell’Angelo et al., 2017).
1) Length to 24 mm (Dell’Angelo et al., 2017 ); seldom exceeds 10 to 12 mm (Kaas & Van Belle, 2003). H/W up to 46% (Kaas & Van Belle, 2003).
2) Side slopes of valves ii to vii as on C. septemvalvis (?). Pleural area on each side of valves ii to vii has 3 to 5 (Kaas & Van Belle, 2003), or 5 to 6 (Capellini, 1859), longitudinal grooves at all growth stages 22 C. septemvalvis . Differentiation from C. septemvalvis is difficult as the grooves are difficult to discern in photographs.
3) Valves, reddish flesh colour at the base; rest of their surface is variable greenish-red (Capellini, 1859) 23 C. septemvalvis .
4) No mention of girdle bands in Capellini, (1859), but on some Mediterranean Callochiton sp.
5) Dorsal surface of girdle has immense quantity of transversely arranged ‘fibres’ [= needles] which are closely appressed to the surface (Capellini, 1859).
6) No mention by Capellini (1859) of minute black aesthete spots on lateral areas of valves.
7) Lateral area has threeslit rays on either side of the intermediate valves (Capellini, 1859).
8) No mention by Capellini (1859) of white apophyses or jugal sinus between them.
9) No mention by Capellini (1859) of gills.
10) Throughout the Mediterranean, Atlantic coasts of Spain near Gibraltar, Canary Islands and Madeira Archipelago, and Morocco (Kaas 1991; west of Cape Yubi). (B. Dell’Angelo, 2023, pers. comm., 19 August).
11) Mucro in the centre of tail valve viii (Dell’Angelo et al., 2017) .
Callochiton stefaniae Dell’Angelo, Renda, Sosso, Sigwart & Giacobbe, 2017
All features derived from Dell’Angelo et al. (2017)
1) Length to 4.6 mm.
2) No longitudinal grooves in the pleural areas.
3) Valve surface uniform very pale brownish to rosaceous.
4) Girdle surface uniform very pale brownish to rosaceous, no pale colour bands.
5) Dorsal surface of girdle has densely packed, transversely arranged, spicules which are shorter (0.075 to 0.09 mm) but, relative to their length, fatter than those of C. septemvalvis and C. doriae..
6) Minute black aesthete spots not detected on lateral areas of intermediate valves.
7) Two slits and slit rays on either side of the intermediate valves, ii to vii, but insertion plates short, teeth irregular and slit rays scarcely visible, even in SEM images.
8) White apophyses on intermediate valves have wider, less shallow jugal sinus between them than on C. septemvalvis.
9) No mention in Dell’Angelo et al. (2017) of gills.
10) Found in Straits of Messina below 75 metres and single record on GBIF in Corsica.
11) Mucro in the anterior part of tail valve viii.
Lepidochitona cinerea (Linnaeus, 1767).
1) Length usually 12 to 16 mm; extreme maximum 28 mm. H/W c. 30% to 40%.
2) Valves ii to vii have convex side slopes 05 C. septemvalvis and no longitudinal grooves in the pleural areas.
3) Dorsal surface of valves has diverse colours and patterns, some of which may resemble those of C. septemvalvis 24 C. septemvalvis .
4) Girdle has diagnostic pattern of alternating dark and light transverse lozenge-like bands.
5) Dorsal surface of girdle has densely packed rounded granules 25 C. septemvalvis .
6) No minute black aesthete spots on lateral areas of intermediate valves. Dorsal surface of valves has numerous rounded, sometimes slightly elongated, granules visible at X5 magnification.
7) Only oneslit and slit ray in the articulamentum on either side of the intermediate valves.
8) White apophyses on intermediate valves are separated by a jugal sinus on valve iv of about same width as one apophysis.
9) Usually 16 to19 gills each side, arrangement holobranch.
10) Only chiton species commonly found higher than MLW on shores of north-west Europe. All round British Isles on rocky shores.
Tonicella marmorea O. Fabricius, 1780.
1) Maximum length 45 mm. H/W c. 37% N.E. Atlantic, c. 44 % N.W. Atlantic (Kaas & Van Belle 2003) c. 20% 05 C. septemvalvis .
2) Valves ii to vii have straight, or nearly straight, side slopes 05 C. septemvalvis and no longitudinal grooves in the pleural areas.
3) Valves have blotches or zigzag lines of dirty white, cream, brown, red and/or ochre. Valves ii and iv often have more dark colour 26 C. septemvalvis .
4) Girdle varies deep purple-red to green and often has many pale transverse bands and small pale blotches on dark areas 27 C. septemvalvis .
5) Dorsal surface of girdle smooth to naked eye; microscope shows sparse, widely spaced, minute granules
6) No minute black aesthete spots on lateral areas of intermediate valves. Dorsal surface of valves has growth lines and numerous small granules which are often not discernible in images.
7) Only one slit and slit ray in the articulamentum on either side of the intermediate valves, ii to vii.
8) White apophyses on intermediate valves are separated by a jugal sinus about a quarter the width of one apophysis.
9) 17 to 26 gills each side, arrangement merobranch.
10) At LWS and sublittorally. Not found further south than North Wales or Northumberland.
Boreochiton ruber (Linnaeus, 1767).
1) Length usually 10 to 15 mm; maximum 21 mm. H/W c. 23% 05 C. septemvalvis .
2) Valves ii to vii have gently convex side slopes 05 C. septemvalvis and no longitudinal grooves in the pleural areas.
3) Dorsal surface of valves pink to brick red with patches and streaks of cream and light brown 28 C. septemvalvis .
4) Girdle usually has alternating dark and pale transverse bands. Pale bands, often fragmentary, usually thinner than dark bands, and roughly in line with shell-sutures 28 C. septemvalvis .
5) Dorsal surface of girdle has densely-packed, elongate c. 0.05 – 0.06 mm, club-shaped granules with packed, touching heads giving a mealy appearance 29 C. septemvalvis .
6) No minute black aesthete spots on lateral areas of intermediate valves. Dorsal surface of valves smooth apart from growth lines. (Faint stippling may be visible at X30.).
7) Only one slit and slit ray in the articulamentum on either side of the intermediate valves, ii to vii.
8) White apophyses on intermediate valves are separated by a jugal sinus on valve iv of about same width as one apophysis.
9) About 12 gills each side (range 10 to15) arrangement merobranch.
10) At LWS and sublittorally. Widespread around Britain except southern North Sea.
Habits and ecology
C. septemvalvis lives, usually in small numbers, on hard substrate, frequently with red algae, on shores at low water spring tide and sublittorally to 500 m (Kaas & Van Belle, 2003). The articulated shell enables it to conform closely to uneven rock surfaces, which the large foot grips firmly. The aragonite shell is weak and frequently fractured, but the valve fragments are held in position by strong muscles which allow the chiton to continue functioning 11 C. septemvalvis . The shell pattern and colours often resemble the varied colours of the rock surface and growths on it 08 C. septemvalvis .
It has no eyes on its head 21 C. septemvalvis but light sensitive intrapigmental aesthetes in the valves 04 C. septemvalvis & 16 C. septemvalvis enable it to react to light and seek shelter and shade under stones when emersed at low water.
Tufts of cilia on the surfaces of the mantle-cavity and gills create an inhalent respiratory water-current entering the mantle-cavity wherever the girdle is raised laterally. The current passes along the cavity through the gills to exit at the posterior under the raised girdle 21 C. septemvalvis .
It feeds by scraping microalgae and associated organisms from the rock surface using its hard radula of chitin mineralized with magnetite. The respiratory water current in the mantle-cavity carries excreta from lateral nephridopores towards the posterior, where faecal pellets from the anus on the anal papilla join the flow to be expelled from under the raised girdle.
Reproduction is dioecious. The water current in the mantle-cavity carries sperm or ova from lateral gonopores to exit at posterior. As fertilization is external, synchronised emission of sperm and ova is needed to ensure success. The ova hatch into planktonic trochophore larvae and later metamorphose into small adult-form young without an intervening veliger stage.
Distribution and status
C. septemvalvis occurs in the north-east Atlantic from 67° N. at Bodö, Norway to Portugal, Spain and the Canary Islands. There are fossil records and unconfirmed reports of it from the Mediterranean (B. Dell’Angelo, 2023, pers. comm., 19 August).
Distinguishing C. septemvalvis from C. doriae is difficult, especially from photographs. No records of C. septemvalvis from the Mediterranean with confirmation by molecular sequencing have been found; most or all records of it may be misidentified C. doriae. GBIF map https://www.gbif.org/species/2306798 . The GBIF map for C. doriae includes records of fossils from north-west Europe.
C. septemvalvis occurs, usually in small numbers, all around the British Isles except in south-east England and north-east Irish Sea where there is turbidity and little hard substrate. NBN map https://species.nbnatlas.org/species/NBNSYS0000174295
Acknowledgements
For use of images I am indebted to Frédéric Andre, Bruno Dell’Angelo, Rob Durrant, David Fenwick, Chris Rickard, Neil Roberts, Vsevolod Rudyi and Michiel Vos. I am most grateful to Paula Lightfoot and Simon Taylor for providing specimens and to Bruno Dell’Angelo and Julia Sigwart for valued help.
References and links
Dell’Angelo, B., Renda, W., Sosso, M., Sigwart, J.D. & Giacobbe, S. 2017. A new species of Callochiton (Mollusca: Polyplacophora) from the Strait of Messina (central Mediterranean). Archiv für Molluskenkunde. 146(2): 243-250., https://doi.org/10.1127/arch.moll/146/243-250
page(s): 244, figs 2-4
Forbes, E. & Hanley S. 1849-53. A history of the British mollusca and their shells. vol. 2 (1853) London, van Voorst. (As Chiton lævis) https://archive.org/details/historyofbritish02forbe/page/411/mode/1up
pp. 411 – 414
Fox, R. 2007. Invertebrate Anatomy On Line. Katharina tunicata http://lanwebs.lander.edu/faculty/rsfox/invertebrates/katharina.html
Jeffreys, J.G. 1862-69. British conchology. vol. 3 (1865). London, van Voorst. (as Chiton laevis Pennant). https://archive.org/details/britishconcholog03jeffr/page/226/mode/2up
Jones, A.M. & Baxter, J.M. 1987. Molluscs: Caudofoveata, Solenogastres, Polyplacophora and Scaphopoda London, Linnean Society, and Estuarine and Brackish-water Sciences Association. (as Callochiton achatinus Brown, 1823 )
Kaas, P. and Van Belle, R.A. 2003. Monograph of living chitons.
vol 2 Leiden – Boston, Brill. [Callochiton septemvalvis pp. 10 – 14]
Matthews, G. circa1954. The identification of British chitons. Papers for Students No.9. London, Conchological Society of Great Britain and Ireland.
Montagu, G. 1803. Testacea Britannica or natural history of British shells, marine, land, and fresh-water, including the most minute: Systematically arranged and embellished with figures. Vol. 1, xxxvii + 291 pp;; Vol. 2, pp. 293–606, pl. 1-16 (as Chiton septemvalvis) https://www.biodiversitylibrary.org/item/78694#page/53/mode/1up (p. 3)
Schwabe, E. 2010. Illustrated summary of chiton terminology. Spixiana 33(2):171-194. https://www.researchgate.net/publication/258843995_Illustrated_summary_of_chiton_terminology_Mollusca_Polyplacophora
Sigwart, J.D., Sumner-Rooney, L.H., Schwabe, E., Heß, M., Brennan, G.P. and Schrödl, M. 2014. A new sensory organ in “primitive” molluscs (Polyplacophora: Lepidopleurida), and its context in the nervous system of chitons. Frontiers in Zoology 2014, 11: 7. http://www.frontiersinzoology.com/content/11/1/7
Sigwart, J.D. and Sumner-Rooney, L. 2021. Continuous and regular expansion of a distributed visual system in the eyed chiton Tonicia lebruni. Biol. Bull. 240: 000-000.
Sigwart, J.D., Stoeger, I., Knebelsberger, T. & Schwabe, E. 2013. Chiton phylogeny (Mollusca: Polyplacophora) and the placement of the enigmatic species Choriplax grayi (H. Adams & Angas). Invertebrate Systematics 27: 603–621.
Glossary
μm = 0.001 mm
aesthetes = complex of canals permeating shell; filled with sensory tissue.
aesthetes, intrapigmental = light sensitive aesthetes lacking a lens, with black pigment within the aesthete but not in surrounding shell matrix (Sigwart & Sumner-Rooney, 2021).
a.k.a. = also known as.
apophysis = (pl. apophyses) anterior extension of articulamentum for attachment of muscles. (a.k.a. sutural lamina).
aragonite = crystalline mineral-form of calcium carbonate; less common on land than calcite, but, currently, the more frequent mineral-form in oceans and living mollusc shells.
articulamentum = inner shell-layer of chiton valves, usually hard, white, porcelaneous aragonite and often differently coloured in central part.
chitin = semitransparent flexible horny protein.
chitinous = (adj.) made of chitin.
cilia (pl.) = motile linear extensions of membrane used in locomotion, or to create water currents in feeding. (“cilium” singular).
dioecious = having separate male and female individuals.
emersed = above water surface, not covered in water.
girdle = peripheral band of thickened, reflexed mantle which encloses ends of valves.
granulose = (of surface) slightly roughened by granules.
holobranch = gills in mantle-cavity extend full length, or almost, of foot.
imbricated = arranged to overlap and alternate like roof tiles.
immersed = covered in water.
insertion plate = extension of articulamentum on lateral margin of valves ii – vii, anterior margin of valve i and posterior margin of valve viii. Inserts into the girdle muscle block.
intermediate valve = valves ii to vii.
jugal = of or at the jugum.
jugum = triangular middle section of central area of intermediate valves. lateral area = (on valves ii – vii) triangular area with its base along lateral edge of valve and its apex near the centre of the posterior edge. a.k.a. lateral triangle.
LWS = low water spring tide, two periods of a few days each month when tide falls lowest.
mantle = sheet of tissue that secretes the shell, surrounds the viscera and forms a cavity for the gill in most marine molluscs.
mantle-cavity = (a.k.a. pallial groove) narrow groove around whole foot and head, roofed by mantle and containing gills, nephridiopores and gonopores.
magnetite = mineral of iron oxide, hardest material made by any living organism (Botelho, 2013).
merobranch = gills in pallial cavity only in posterior two-thirds of animal.
motile = (adj.) able to move spontaneously; applicable to whole animals or to parts of them such as cells, gametes or cilia.
mucro = projection on tail valve (viii) of chiton demarking posterior from rest of valve; varies in prominence and position.
nephridium = tubular excretory/osmoregulatory organ. (a.k.a. kidney).
nephridiopore = opening of nephridium for excretion. (a.k.a. nephropore, or renal pore).
pleural area = triangular area with its base along anterior edge of intermediate valve and its apex on the posterior edge. (a.k.a. median triangle).
plankton = animals and plants that drift in pelagic zone (main body of water).
quincunx = pattern of five dots as on playing cards and dice.
radula = ribbon of chitin bearing chitinous teeth impregnated with magnetite.
SEM = scanning electron microscope; produces images by scanning the surface with a focused beam of electrons.
side slope = shape in profile view (from posterior or anterior) of lateral areas of intermediate valves; may be straight, convex, concave or a combination of these.
sinus = a notch, depression or cavity on the surface.
slit ray = row of canal pores running diagonally from lateral slit to posterior. edge on ventral surface of chiton valve (a.k.a. notch ray).
suture = (of chiton) line where two valves meet.
tegmentum = outer shell-layer of chiton valves, usually porous and relatively soft.
trochophore = spherical or pear-shaped larvae that move with aid of girdle of cilia. Subsequent veliger stage of most marine molluscs is not found in chitons.
veliger = shelled larva of marine gastropod or bivalve mollusc which swims by beating cilia of a velum (bilobed flap).
WoRMS = World Register of Marine Species