Click image to enlarge with full caption. Main text below slider.
Elysia viridis (Montagu, 1804).
Authors: Ian F. Smith (text) and Malcolm Storey (shore work and photography).
PDF available at www.researchgate.net/publication/352311905_Elysia_viridis….
Current taxonomy; World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=139686
Synonyms: Laplysia viridis Montagu, 1804.
GLOSSARY below.
Description
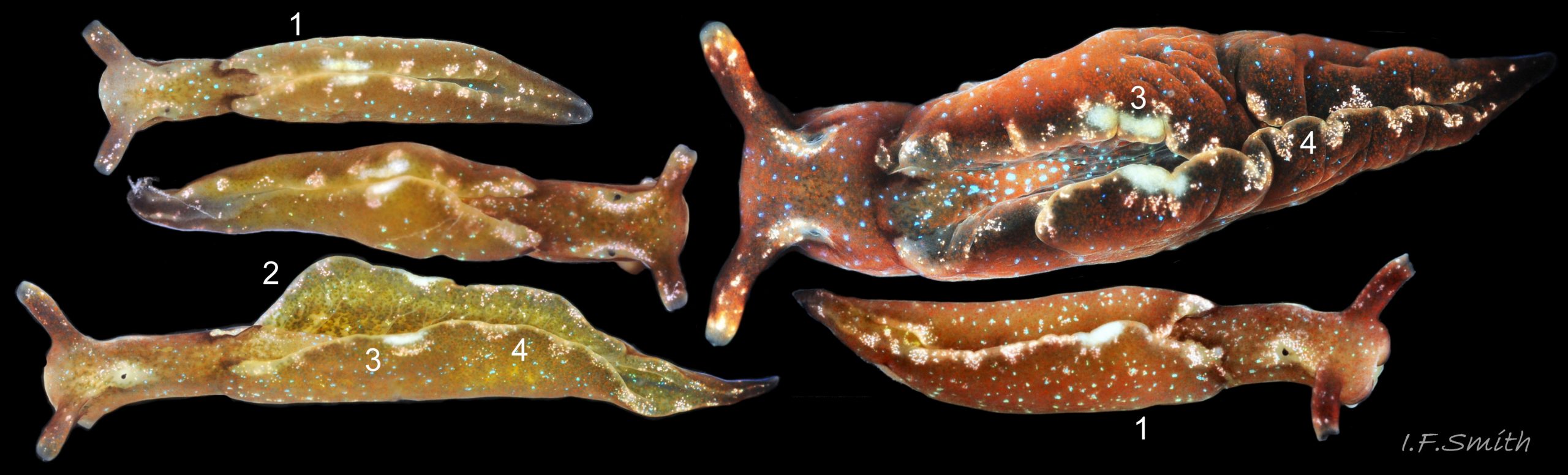
The smooth body, lacking tubercles, has a usual maximum length in Britain of 45 mm with the large head occupying the anterior 25% ( 01 Elysia viridis ). The rear 75% is flanked by large parapodial lobes which can be closed over the body or opened widely ( 02 Elysia viridis) exposing their upper surface and dorsum of the body.
A deep groove separates the head laterally and ventrally from the rest of the body ( 03 Elysia viridis). The head has a pair of tightly enrolled rhinophores ( 04 Elysia viridis) which start to appear when the body is about 3 mm long. There are no oral tentacles. The anterior of the head has a central cleft ( 05 Elysia viridis). Laterally behind each rhinophore there is a small black eye in a pale area ( 03 Elysia viridis) bordered by freckles of white pigment which continue at varying densities onto the rhinophores. The radula is reduced to a single row of teeth adapted solely for slitting and cutting (Taylor, 1968). The most usual ground colour of the body is some shade of brown or olive, often with a green or red cast. Those feeding on Codium are usually dull olive green ( 06 Elysia viridis ) but colours can include bright green ( 02 Elysia viridis & 08 Elysia viridis), red-brown ( 01 Elysia viridis ), orange and cream ( 05 Elysia viridis). They are variably speckled with glistening blue, turquoise or green ( 07 Elysia viridis, 09 Elysia viridis & 10 Elysia viridis). When the parapodial lobes are spread open, their inner surfaces and dorsum of the body are often green with a visible leaf-like ( 02 Elysia viridis & 08 Elysia viridis) dendritic digestive gland. The rim of each lobe usually has an opaque white mark ( 02 Elysia viridis ), often with several other less distinct whitish marks ( 01 Elysia viridis).
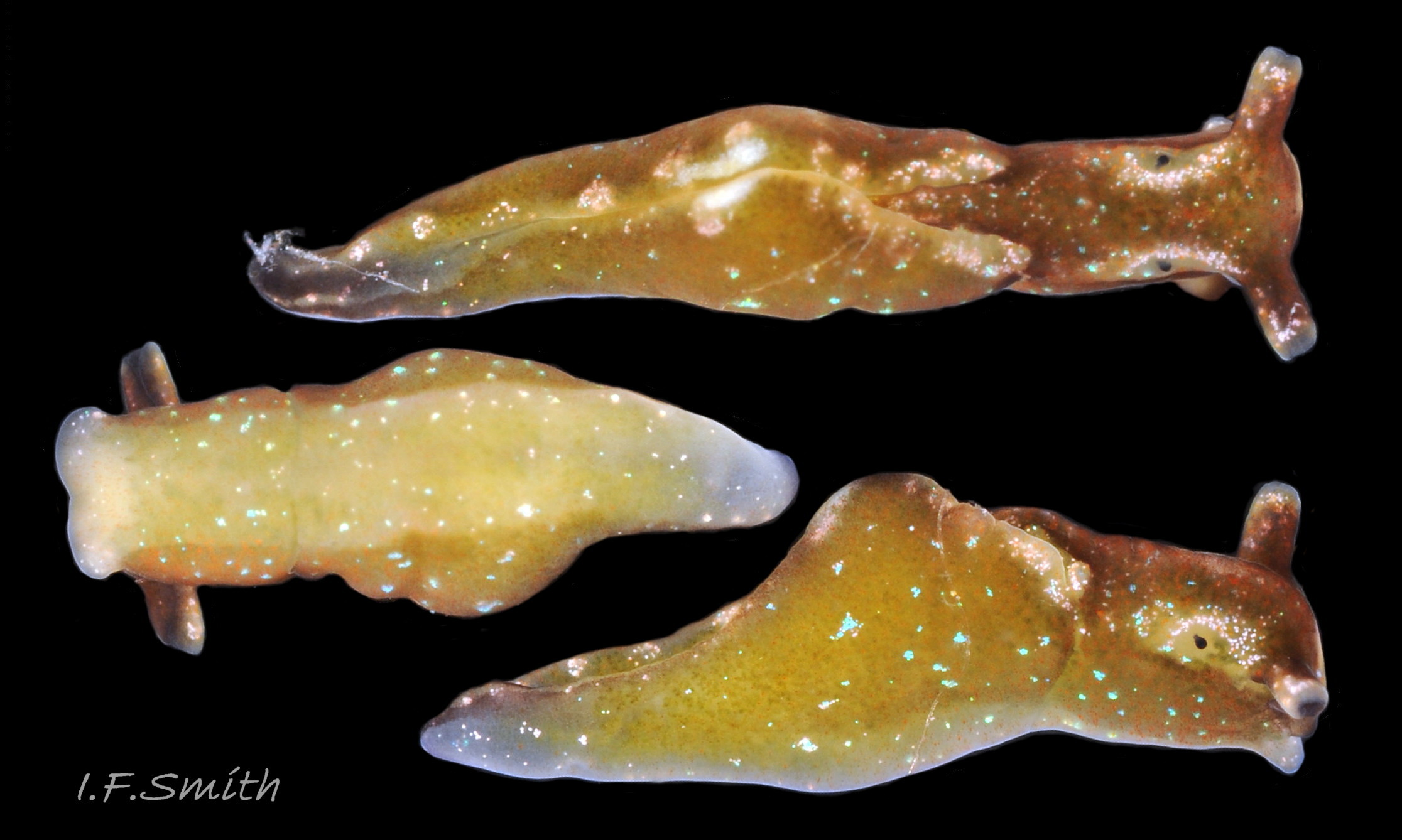
The anterior of the foot is rounded and expanded ( 12 Elysia viridis) but rarely extended into distinct curved propodial tentacles as drawn by Meyer & Möbius, (1865 in Thompson, 1976). The translucent pale sole shows the variable colour of the digestive gland and other viscera ( 11 Elysia viridis) and often has many white, pinkish flakes and/or fine brown pigment specks (figs 3 03 Elysia viridis & 12 12 Elysia viridis).
Key identification features
E. viridis
1) Large parapodial lobes ( 02 Elysia viridis), start to form before 3 mm body length ( 04 Elysia viridis).
2) Speckled with glistening blue, green or turquoise ( 09 Elysia viridis & 10 10 Elysia viridis).
3) No oral tentacles or enrolled oral veil.
4) Usual maximum length in Britain 45 mm.
Similar species
Aplysia punctata (Cuvier, 1803)
1) Large parapodial lobes ( 13 Elysia viridis).
2) Not speckled with glistening blue or turquoise.
3) Oral veil enrolled to resemble large oral tentacles (14 Elysia viridis).
4) Usual maximum length 120 mm.
Habits and ecology
E. viridis lives on the lower shore and in the shallow sublittoral where there is enough light for its food algae. The single row of radular teeth, adapted to only slitting and cutting (Taylor, 1968), restricts E. viridis to suctorially feeding from algae with few or no internal cell walls subdividing the cytoplasm. The leading tooth is used to puncture algal cell walls whereas the newer, unused teeth function as a spear shaft, and the older worn out teeth are retained in a coil (C.D. Trowbridge 2021, pers. comm., 16 May). Suitable algae in north-west Europe include the siphonaceous green Codium ( 15 Elysia viridis and Bryopsis ( 16 Elysia viridis) and the coenocytic green Cladophora ( 17 Elysia viridis) and Chaetomorpha and red Griffithsia, Halurus, Dasya, and Dasysiphonia (Trowbridge, 2010; van Bragt, 2004 and C.D. Trowbridge 2021, pers. comm., 9 May). Other coenocytic and siphonaceous species may be consumed when locally available. These vary geographically and with the dates of local invasion by suitable alien algal species.
Early publications (Forbes & Hanley, 1853 and Jeffreys, 1869) mentioned E. viridis on the obviously unsuitable vascular plant Zostera which probably had suitable algae growing among or on it. Accurate identification of which precise species and subspecies of algae are consumed often requires close microscopic examination.
In Britain, the most frequently recorded food alga is ‘Codium’ which includes (Brodie et al., 2007) the native species C. tomentosum and C. vermilara, the invasive (since 1953 in Scotland) alien C. fragile subsp. fragile, previously referred to as C. fragile subsp. tomentosoides and the less common, native or long established (since 1826 in Scotland) alien, C. fragile subsp. atlanticum. These species and subspecies are difficult for recorders to differentiate and there are many misidentified records, but E. viridis can distinguish them as they find the thinner utricle walls of the common alien C. fragile subsp. fragile easier to penetrate than in the others. The slugs have a marked preference for it whenever it is available, and their associated growth rates and maximum body size are greater than when other algal species are consumed (Trowbridge & Todd, 2001). In contrast, of 886 thalli examined of C. fragile subsp. atlanticum from eleven sites all around Scotland, not one had E. viridis on it (Trowbridge & Todd, 2001).
Historically, E. viridis may have frequently fed on Cladophora (01 Elysia viridis & 17 Elysia viridis) but it is now only rarely used at sites where the preferred alien C. fragile subsp. fragile is still absent (pers. obs. and Trowbridge & Todd, 2001). Experiments showed that those born from adults feeding on the alien lacked the ability to feed or grow on Cladophora. There may have been a historic host shift from Cladophora to Codium fragile subsp. fragile (Trowbridge & Todd, 2001).
Body colour appears to vary with the algal species ingested; dull olive-green with Codium ( 06 Elysia viridis), greenish with other green algae and reddish-brown with most red algae ( 01 Elysia viridis). Van Bragt (2004) correlated in the Oosterschelde, Netherlands, cream ( 05 Elysia viridis) with Dictyota dichotoma and pink, red or orange ( 05 Elysia viridis) with the alien Dasysiphonia.
Chloroplasts are sequestered from the ingested cytoplasm of Codium spp. and continue photosynthesis for less than 24 hours within the slug’s body. They may be of a small but significant benefit to the animal as symbiotic organelles if constantly renewed by feeding (Taylor, 1968). It is not known if this phenomenon applies to other algal host species (Trowbridge & Todd, 2001).
There is no record of the large, mobile parapodial lobes being used by E. viridis in active swimming, but they assist when it drifts on currents, and their large surface may assist respiration or short term photosynthesis of ingested chloroplasts in the dendritic digestive gland visible in the surface.
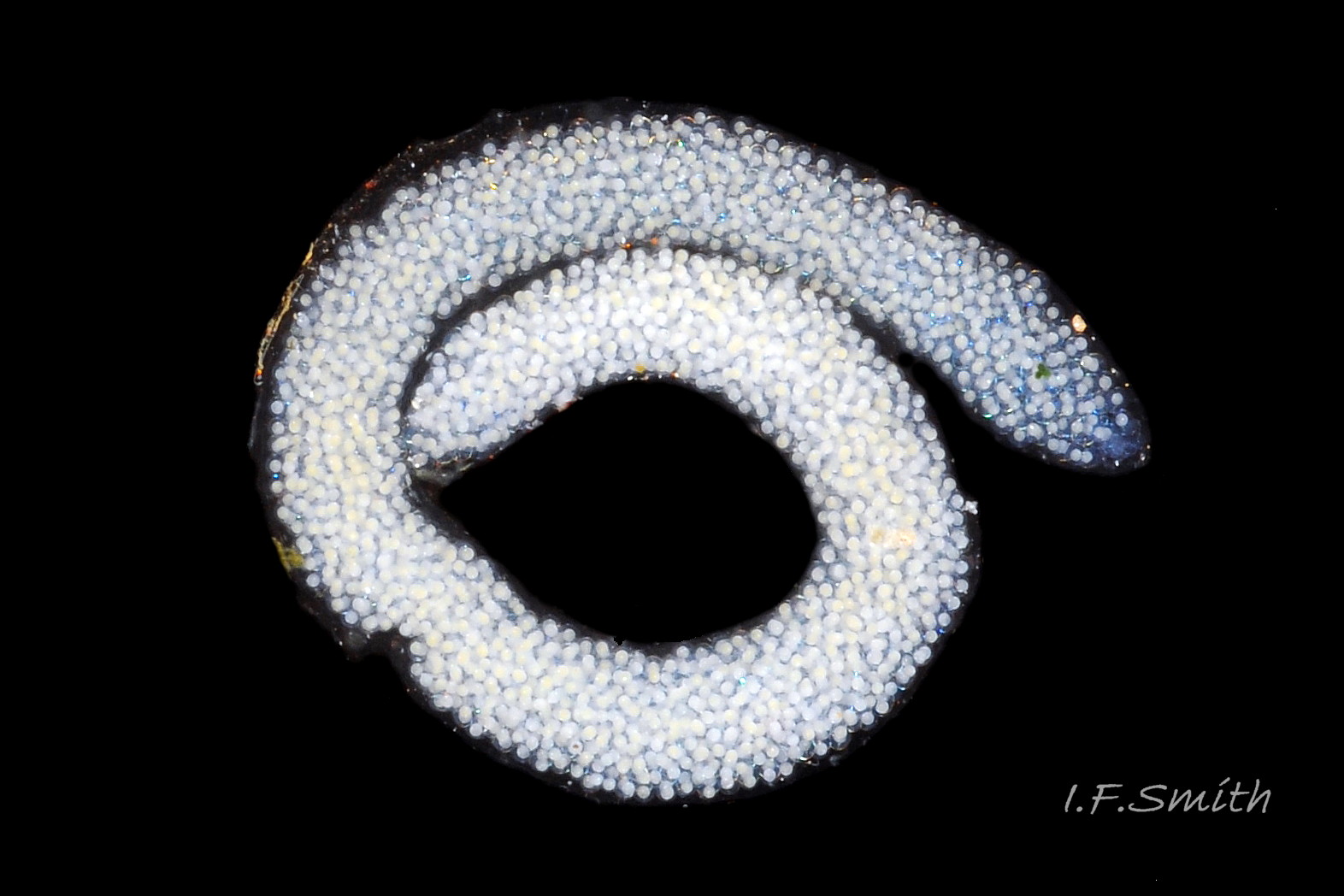
Spawn is deposited on food algae ( 06 Elysia viridis) in north-west Europe from May to October, when the mean monthly water temperature is above 10° C (Rasmussen, 1973). When on a flat surface, it forms a spiral cord of about one and a half turns, diameter about 5.5 mm ( 18 Elysia viridis), containing over 800 ova (Rasmussen, 1973). Spawn colour is reported to vary with the algal species eaten by the adult; reddish-yellow for Chaetomorphum linum in Denmark (Rasmussen, 1973), lemon-yellow to bluish white for Cladophora and white for Codium (Trowbridge & Todd, 2001), but variation in hue with age is also likely. After 5 to 12 days, planktonic larvae emerge for a long larval stage of 30 to 46 days at 15° C (Trowbridge & Todd, 2001) before settling and metamorphosing on a food alga. Lifespan is 12 to 15 months. The length when fully grown varies geographically from a norm of about 27 mm in the Mediterranean to 45 mm in north-west Europe with an extreme specimen of 70 mm in the Netherlands (Trowbridge & Todd, 2001). Within a given area, length is probably affected by diet, being less on the native Cladophora than the aliens Codium fragile subsp. fragile and Dasysiphonia (to 70 mm), but uncontrolled variables at diverse sites render quantitative comparisons uncertain.
Distribution and status
E. viridis occurs from Shetland and Norway into the Mediterranean and into the Baltic as far as Kiel, GBIF map www.gbif.org/species/5192088 . It is widespread around Britain and Ireland, and locally common except in the North Sea which has few records on the NBN UK map species.nbnatlas.org/species/NBNSYS0000175103 . Jeffreys (1869) commented on the absence of records from the North Sea, and extensive fieldwork and search of historic records produced no record of it on the Scottish coast of the North Sea in McKay & Smith (1979). Almost all North Sea records of E. viridis on NBN Atlas, including some for Scotland, are post 2000 except for two in North Yorkshire (C. Todd, 1975 and K. Hiscock, 1993). It appears that since 2000 its population has increased in the North Sea from absence in Scotland and very low numbers in England to a noticeable presence ( 19 Elysia viridis. ). If not because of increased recording and reporting online by divers, this increase may be due to recent warming of the North Sea (Hughes et al., 2010) which is colder in winter than other seas around Britain. This would accord with the situation in the Netherlands. It was first recorded there in 1899 but was absent 1938 – 1989, recovered until locally wiped out in the severe winters of 1995/96 and 1996/97 and reappeared in 1998 in the Oosterschelde to become one of the commonest sea slug species in that estuary by 2004 (van Bragt, 2004).
Acknowledgements
I am most grateful to Cynthia D. Trowbridge for her help and advice with the text, but any errors or omissions are my (IFS) responsibility.
I thank Rokus Groeneveld www.diverosa.com/nederland.htm , Penny Martin, Chris Rickard, Malcolm Storey www.bioimages.org.uk/ and Stefan Verheyen for use of their images and Peter H. van Bragt for help with literature.
References and links
AlgaeBase Codium fragile (Suringar) Hariot 1889 accessed 10 May 2021 www.algaebase.org/search/species/detail/?species_id=3638
Brodie, J. A., Maggs, C. and John, D. M. (eds.). 2007. Green Seaweeds of Britain and Ireland. British Phycological Society.
Forbes, E. & Hanley S. 1853. A history of the British mollusca and their shells. vol. 3, London, van Voorst. archive.org/details/historyofbritish03forbe/page/614/mode…
Garstang, W. 1890. A complete list of the Opisthobranchiate Mollusca found at Plymouth. J. mar. biol. Ass. U.K., 1:399–457.
plymsea.ac.uk/id/eprint/50
Hughes, S.L., Holliday, N.P., Kennedy, J., Berry, D.I., Kent, E.C., Sherwin, T., Dye, S., Inall, M., Shammon, T. and Smyth, T. 2010. Temperature (Air and Sea) in MCCIP Annual Report Card 2010-11, MCCIP Science Review, 16pp. www.mccip.org.uk/arc
Jeffreys, J.G. 1869. British conchology. vol. 5 . London, van Voorst.
archive.org/details/britishconcholog05jeffr/page/31/mode/1up
Taylor, D.L. 1968. Chloroplasts as symbiotic organelles in the digestive gland of Elysia viridis (Gastropoda, Opisthobranchia). J. mar. biol. Ass. U.K., 48 (1): 1 – 15. Abstract: www.cambridge.org/core/journals/journal-of-the-marine-bio…
Thompson, T.E. 1976. Biology of opisthobranch molluscs 1. London, Ray Society.
Trowbridge, C. D., Hirano, Y. J. and Hirano, Y. M. 2010. Sacoglossan opisthobranchs on northwestern Pacific shores: Stiliger berghi Baba, 1937, and Elysia sp. on filamentous red algae. Veliger 51: 43-62. www.researchgate.net/publication/235703273_Sacoglossan_Op…
Trowbridge, C. D, and Todd, C. 2001. Host-plant change in marine specialist herbivores: ascoglossan sea slugs on introduced macroalgae. Ecological Monographs, 71 (2): 219–243. Ecological Society of America.
www.researchgate.net/publication/250075515_Host-Plant_Cha…
Van Bragt, P. H. 2004. The sea slugs, Sacoglossa and Nudibranchia (Gastropoda, Opisthobranchia), of the Netherlands. Vita Malacologica, 2: 3 – 32 and Pl. 1 -10.
Current taxonomy; World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=139686
GLOSSARY
chloroplast = organelle in the cytoplasm of a plant or algal cell which contains chlorophyll that photosynthesises to capture and store the energy from sunlight.
coenocytic = (of algae) with parts made up of multinucleate, large masses of cytoplasm enclosed by the wall of each large cell.
dendritic = branching like boughs, branches and twigs of a tree.
parapodial lobes = flaps of the parapodium, lateral outgrowths of foot, which extend up the sides of some sea slugs.
propodial = at the front of the foot.
radula = chitinous ribbon of teeth
rhinophore = chemo-receptor tentacle; nudibranch and most sacoglossan sea slugs have a pair on top of the head.
siphonaceous = (of algae) entire thallus (‘plant’) is coenocytic with no internal cell walls subdividing the cytoplasm.
utricle = swollen cortical sac-like portion of filaments in Codium and many related green algae.
vascular plants = plants which, unlike algae, have vascular tissues to transport water and nutrients through the plant, true absorptive roots and leaves specialized in photosynthesis. Usually terrestrial or in freshwater; a few, such as Zostera, live in the sea.
veliger = shelled larva of marine gastropod or bivalve mollusc which moves by action cilia on a velum (bilobed flap).