Click image to enlarge with full caption. Main text below slider.
Facelina bostoniensis (Couthouy, 1838)
PDF version available at www.researchgate.net/publication/358694348_Facelina_bosto…
Current taxonomy: World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=139908
Synonyms: Eolis bostoniensis Couthouy, 1838; Coryphella bostoniensis (Couthouy, 1838); Eolis curta Alder & Hancock 1843; Eolis drummondi W. Thompson, 1844; Eolis tenuibranchialis Alder & Hancock, 1845.
GLOSSARY BELOW
Description
Facelina bostoniensis grows up to 55 mm long; its length when extended is about 4 X its width. The translucent white, or faintly brownish white, main body reveals some reddish and pinkish internal organs 01 Facelina bostoniensis . The only surface pigment is white which may iridesce blue, but blue is less frequent than on F. auriculata. The medial line of white or “bright azure” (Couthouy, 1838) on the tail may be strongly developed 02 Facelina bostoniensis or indistinct 03 Facelina bostoniensis . Nearly always (Thompson & Brown, 1984), there is a streak or some spots of white pigment between and in front of the rhinophores 01 Facelina bostoniensis & 02 Facelina bostoniensis .
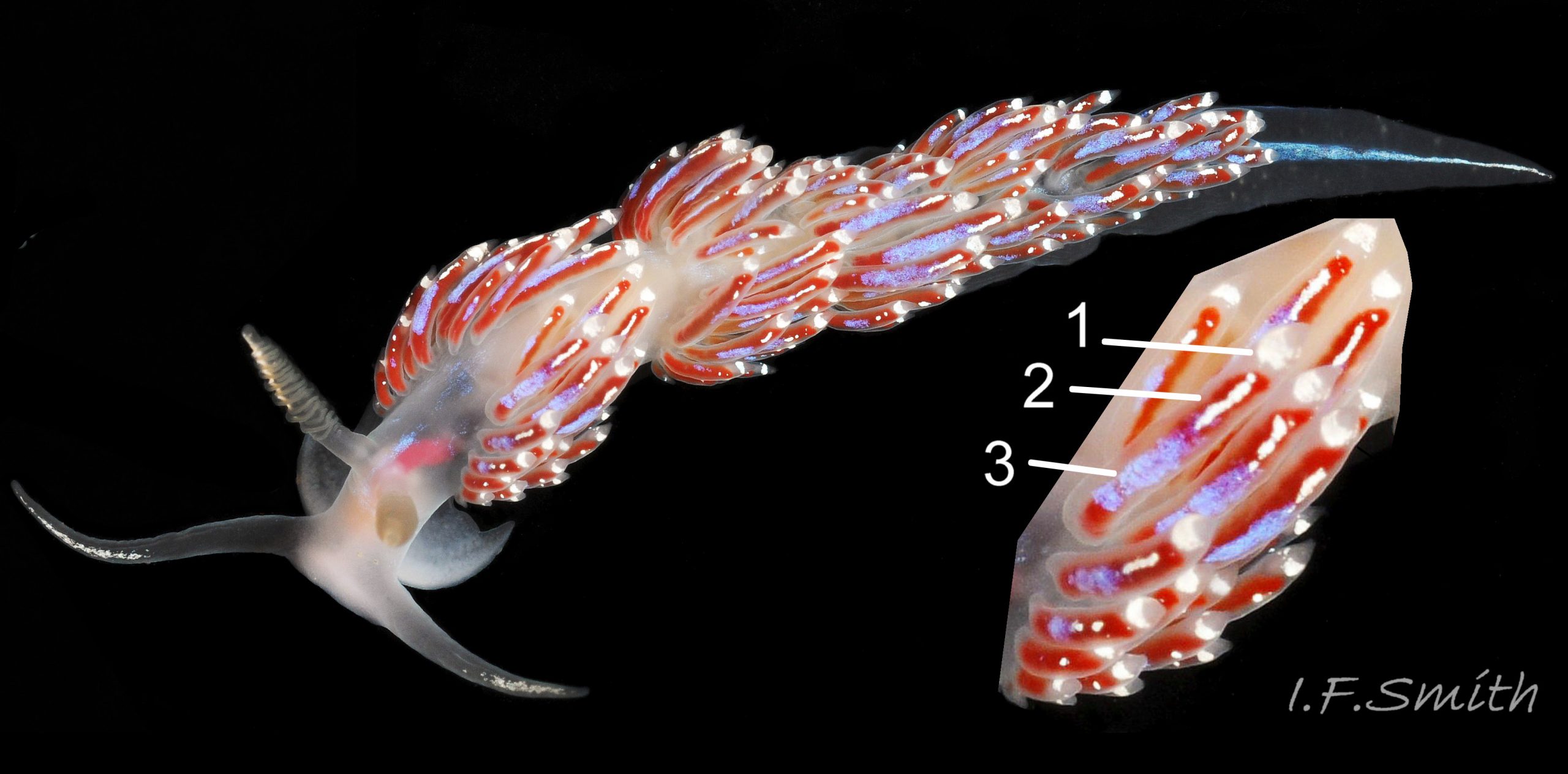
Many of the long linear cerata are between 33% and 50% of the body length (Thompson & Brown, 1984). They are usually held loosely, often untidily, at a variety of angles from upright 04 Facelina bostoniensis to prone 02 Facelina bostoniensis . Their bases 05 Facelina bostoniensis are grouped in poorly defined clumps which are usually obscured by the long cerata overlapping each other. The clumps are most discernible on juveniles about 5 mm long which have fewer cerata to obscure the view 03 Facelina bostoniensis . Sometimes the anterior clump is recognisable on adults when the cerata are curved over the body 06 Facelina bostoniensis & 07 Facelina bostoniensis , but the clumps are rarely as compact as on F. auriculata.
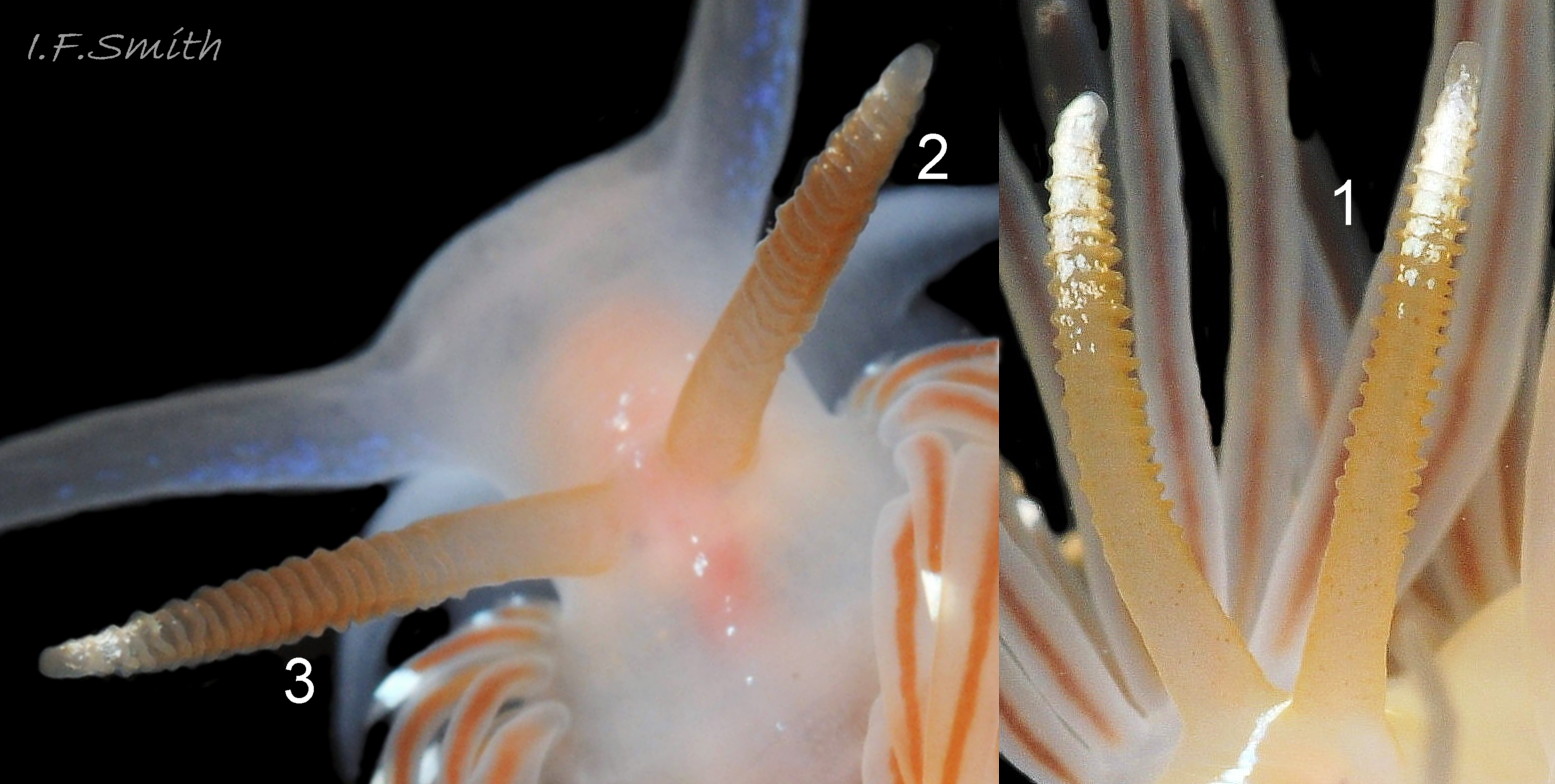
The cerata are translucent whitish, revealing a yellow or light reddish brown to dark chocolate brown 08 Facelina bostoniensis internal digestive gland which varies with diet (Anderson 2022). Whitish cnidosacs may be clearly visible inside the nearly transparent distal end on juveniles 09 Facelina bostoniensis , but on adults they are mostly concealed by a subapical, opaque white band which is narrow at the posterior and shield-shape at the anterior 10 Facelina bostoniensis . The shield extends a short distance down the anterior of the cerata, but there are no white pigment splashes on the rest of the ceratal surface, just the occasional minute white spot or two 02 Facelina bostoniensis . The cerata are the least likely of the upper surfaces of F. bostoniensis to have blue iridescence, but even they have it occasionally 11 Facelina bostoniensis . There seems to be an association of such instances with depth and places open to clean Atlantic Ocean water in Norway and west Ireland, but molecular sequencing might reveal them to be other species.
The rhinophores have up to up to thirty lamellae coloured rosy fawn to yellowish brown 12 Facelina bostoniensis on adults, and whitish 09 Facelina bostoniensis on juveniles. The translucent stem is paler, sometimes white 08 Facelina bostoniensis , at the base and translucent white apically. The distal quarter has opaque white pigment, mainly on the anterior 12 Facelina bostoniensis .
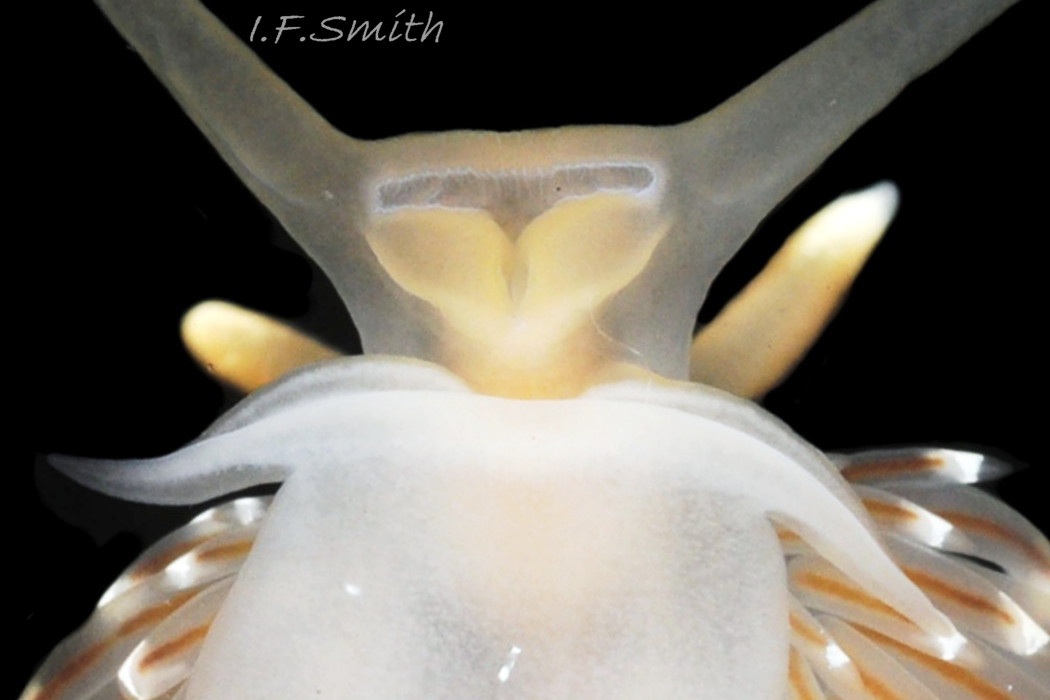
The ventral surface of the head consists of two large, flat, fleshy lips which open along T-shaped slits 13 Facelina bostoniensis & 14 Facelina bostoniensis for the inner lips with mouth and jaws to be brought forward to cut the prey. The very long, broad-based oral tentacles taper to a fine point. On adults they are about twice as long as the rhinophores 04 Facelina bostoniensis & 06 Facelina bostoniensis , and on juveniles they can be half the length of the body 03 Facelina bostoniensis . They are translucent white or whitish, usually with some opaque white pigment on the distal third or half and, sometimes, some blue iridescence. Couthouy (1838) described them as “smooth, of a lake [presumably ‘blue lake’] colour and beautifully tinged in the middle with azure”.
The foot is translucent white both dorsally 15 Facelina bostoniensis and on the sole 16 Facelina bostoniensis . It is broad at the anterior, tapering gradually 16 Facelina bostoniensis or rapidly 17 Facelina bostoniensis , depending on its extension, to a fine posterior point. There is usually a white dorsal line on the metapodium, which may iridesce “bright azure” (Couthouy,1838) 02 Facelina bostoniensis . There are prominent, slender, curved propodial tentacles; they and the anterior of the foot, which they are continuous with, are bilaminate 14 Facelina bostoniensis . The propodial tentacles are often folded closely against the foot 13 Facelina bostoniensis . Couthouy (1838) seems to have regarded them as “labial or oral appendages – – – which are entirely separate from the foot and extend across it and beyond it”; 4 14 Facelina bostoniensis shows that they are part of the foot and not of the head or mouth.
Internal anatomy may be visible through the translucent body.
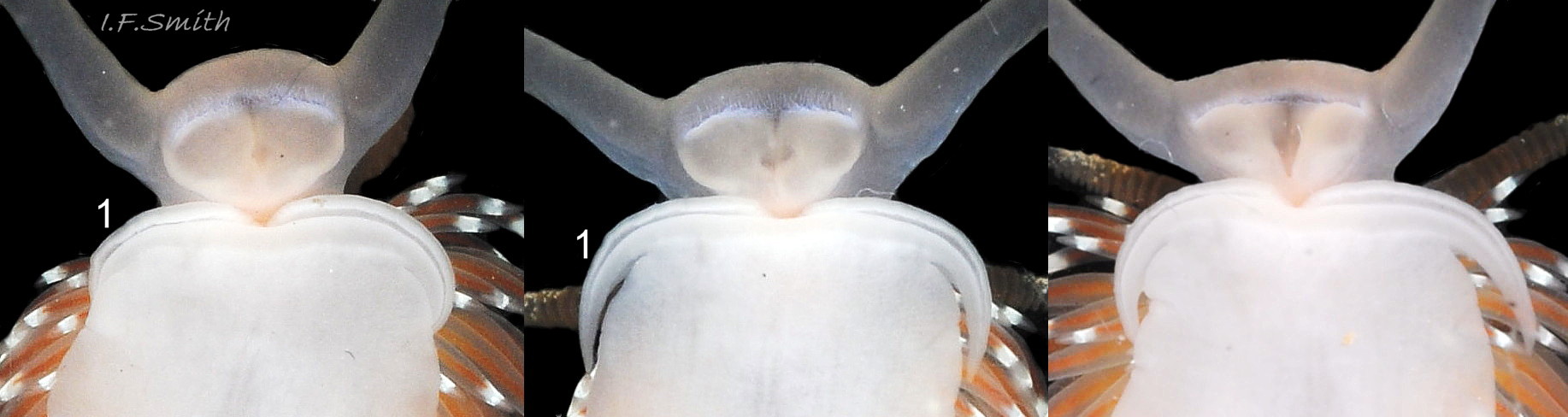
An internal black eye at the base of each rhinophore may be faintly discernible on adults 18 Facelina bostoniensis and is more visible on the more transparent juveniles 09 Facelina bostoniensis .
There is a reddish (fawn on juveniles) oesophagus behind the rhinophores and a pinkish buccal mass at variable distances in front of them 19 Facelina bostoniensis & 20 Facelina bostoniensis .
The stomach, when filled with food, may be seen behind the oesophagus 03 Facelina bostoniensis .
A pulsating mound may be visible on the dorsum over the pericardium containing the heart 21 Facelina bostoniensis and 11 Facelina bostoniensis .
Key identification features
Facelina bostoniensis
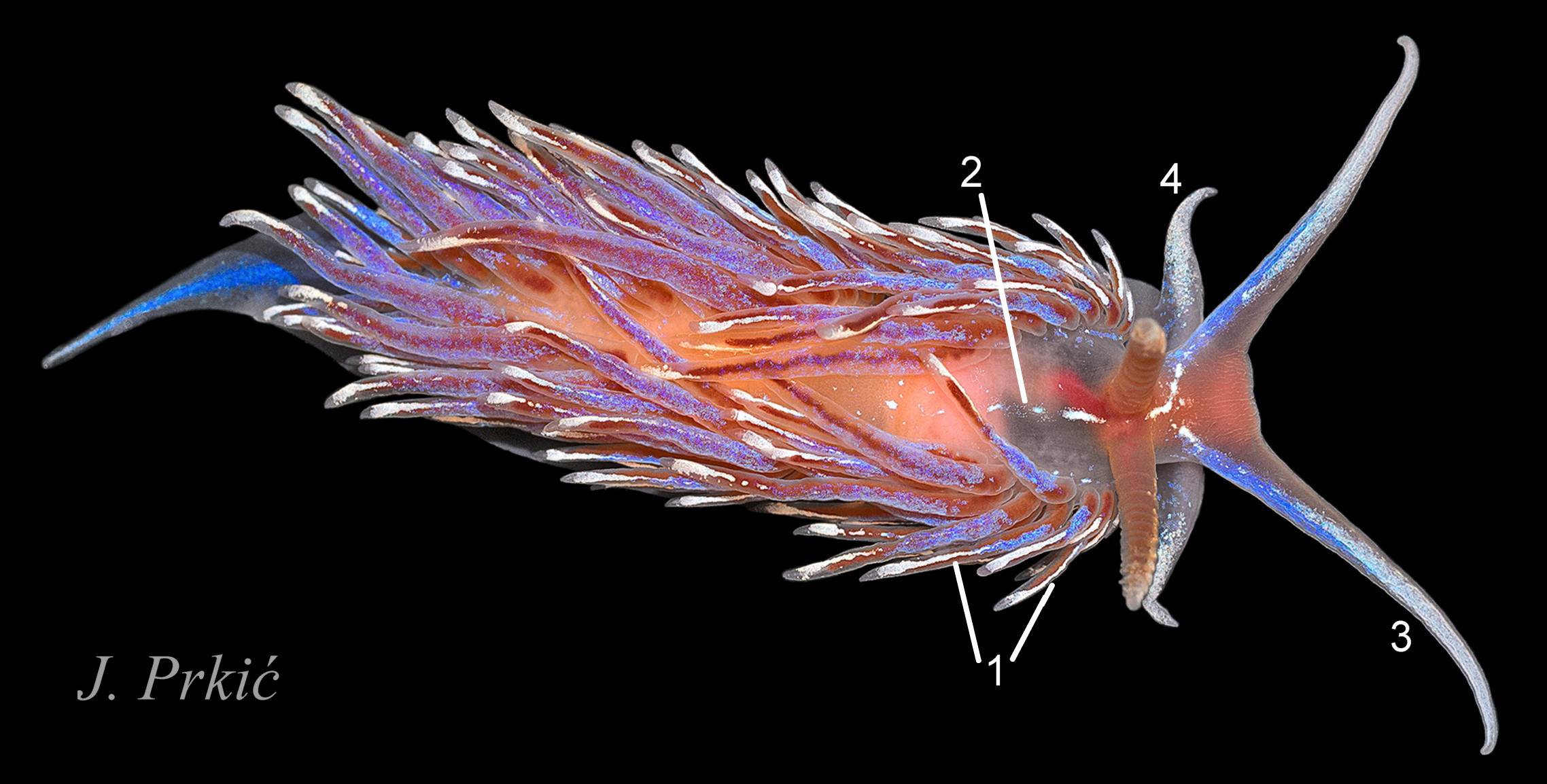
The most consistent diagnostic feature is ‘4’.
1) Body broad, when extended, length is about 4 X width 22 Facelina bostoniensis
2) Many of the linear cerata are between 33% and 50% of the body length and are held loosely at various angles. Poorly defined clumps are obscured by overlapping. 02 Facelina bostoniensis & 04 Facelina bostoniensis .
3) Usually no blue iridescence on cerata 08 Facelina bostoniensis , but quite frequently some on other parts (Couthouy, 1838) 01 Facelina bostoniensis and blue cerata do occur, especially at depth in clean water in west Ireland and Norway 11 Facelina bostoniensis . (Molecular investigation of the latter is desirable.)
4) Subapical, opaque white band on cerata is narrow at posterior and shield-shape at anterior. No white pigment splashes down cerata, but sometimes a very few small, white spots 02 Facelina bostoniensis .
5) The lamellated rhinophores nearly always have a streak or some spots of white pigment between and in front them 12 Facelina bostoniensis .
6) Well developed, slender, flexed propodial tentacles 22 Facelina bostoniensis.
7) Found on both sides of the North Atlantic, possibly not in the Mediterranean.
Similar species
Facelina auriculata (Müller, 1776)
The most consistent differentiating feature is ‘4’.
1) Slender body, when extended, length is about 8 X width 22 Facelina bostoniensis.
2) Cerata rarely more than 25% of body length so only slightly overlap adjacent clumps, which are usually neat, compact and more clearly defined than on F. bostoniensis 23 Facelina bostoniensis.
3) Usually, but not always, some blue iridescence on cerata and, often, on head and body 23 Facelina bostoniensis.
4) Subapical, opaque white band on cerata is amorphous, not shield-shaped. Frequently, there are white pigment splashes down the front of cerata 23 Facelina bostoniensis.
5) The lamellated rhinophores never have a streak or spots of white pigment between them (Thompson & Brown, 1984), unless a specimen with lots of general pigment marks.
6) Propodial extensions much shorter and less slender than on F. bostoniensis 22 Facelina bostoniensis.
7) Found in Europe from northern Norway to the western Mediterranean, not in America.
Facelina vicina (Bergh, 1882)
Features below, based on / calculated from Bergh (1882), are of a single 40 mm, live specimen of F. vicina (lost holotype) after a journey from Trieste to Copenhagen, so features may not have been fully extended, and intraspecific variation should be expected. Recent molecularly sequenced finds from Croatia (like Trieste, in the northern Adriatic) showing the range of intraspecific variations are in 26 Facelina bostoniensis , 27 Facelina bostoniensis, 28 Facelina bostoniensis and 28 Facelina bostoniensis.
The most consistent differentiating feature is ‘4’.
1) Broad body, length 4.7 X width.
2) Cerata up to 9 mm long, 22.5 % of body length. [ 32% to 58% on Croatian specimens].
3) Dorsum of tail, proximal half of oral tentacles and anterior face of cerata have bluish-silver [iridescence]. [Variable extent and intensity on Croatian specimens; a few have none.]
4) Cerata have a chalky-white tip. [Recent Croatian, molecularly sequenced finds also have an opaque white line or dots running from the white tip to about half way down the cerata.]
5) The lamellated rhinophores have a median row of discrete, elongated white spots between and behind them, almost to the pericardium. [Length varies in Croatia. On some the line also projects forwards from the rhinophores.]
6) Propodial tentacles protrude 3 mm each side of foot. [Similar lengths, relative to body size, on Croatian specimens.]
7) Only known from the Mediterranean and its branches. Identifications from NW Europe relying on iridescence alone are not sufficient as all three species have widely variable amounts. Molecular sequencing is needed to ascertain if it occurs in the Atlantic.
Other features of F. vicina
Almost colourless body shows viscera, such as the elongated, yellow-white hermaphrodite gland visible through the posterior half of the sole.
Bright rosy red head, particularly intense on the front and under the neck. [Missing from some Croatian specimens.]
Long oral tentacles nearly twice as long as rhinophores and 25% body length. [Longer on some Croatian specimens.]
Ecology and behaviour
F. bostoniensis lives on the lower part of rocky shores, and sublittorally. Its preferred prey is Tubularia species, but it will eat a range of hydroids, other nudibranchs, or even pieces of mussel when hungry. It defends itself by the release of stinging cnidocytes sequestered from its prey and stored in cnidosacs at the tips of the cerata 10 Facelina bostoniensis .
Like other nudibranchs, it is a simultaneous hermaphrodite. The spawn is a colourless, transparent string containing many short, curved, white sections of ova separated by small gaps of invisible string. Each spawn mass contains about 8000 ova (Thompson & Brown, 1984). It is deposited on hydroid or the substrate in a Grecian key spiral according to Alder and Hancock (1845-55) but photographs show an untidy mass resembling a coil of white sausages, perhaps due to changes after deposition 24 Facelina bostoniensis .
Distribution and status
F. bostoniensis is recorded from Nova Scotia and New England 25 Facelina bostoniensis , and from southern Norway to Atlantic Spain. Nearly all European records on the GBIF map www.gbif.org/species/2292199 (accessed 8 February 2022) are further north than Brittany.
The molecular sequence of a single specimen from New Hampshire, USA, (Gen Bank KY129046) is only 1.67% different from that of three specimens from Scotland (AY345031 & KY513635) and Sweden (KY513637), and is insufficient to challenge acceptance of F. bostoniensis as an amphiatlantic species (Carmona, 2020).
The molecular sequences of Facelina sp. specimens from Croatia fig 26 26 Facelina bostoniensis and Mediterranean France 30 Facelina bostoniensis. differ by 8% from the Scottish and Swedish specimens (Carmona, 2020). This supported the proposal by J. Prkić that they are a separate Mediterranean species, which Carmona (2020) considered to be Facelina vicina (Bergh, 1882) and hypothesised that previous records of F. bostoniensis in the Mediterranean are misidentifications of F. vicina. The hypothesis needs testing with further widespread sequencing of Mediterranean specimens.
F. bostoniensis is widespread around Britain and Ireland; in Scotland it is notably common on the west coast and scarce on the east coast (McKay & Smith, 1979). UK distribution map NBN species.nbnatlas.org/species/NBNSYS0000175326
Acknowledgements
I am indebted to Simon Taylor for providing specimens for photography; this account would not have been possible without them. For their generous contribution of most useful images, I thank Jim Anderson, Jeff Goddard, Josep Lluis Peralta, Jakov Prkić and Erling Svensen. For information and literature concerning American material I thank Karin Fletcher and Jeff Goddard.
References and links
Alder, J. and Hancock, A. 1843. Notice on a British species of Calliopaea d’Orbigny and on four new species of Eolis with observations on the development and structure of the nudibranchiate mollusca. Annals and Magazine of Natural History. 12: 233-236. (As Eolis curta). www.biodiversitylibrary.org/page/22101138#page/248/mode/1up
Alder, J. & Hancock, A. 1845-1855. A monograph of the British nudibranchiate mollusca. London, Ray Society. Family 3 Plate 13. (As Eolis drummondi). www.biodiversitylibrary.org/item/131598#page/334/mode/1up
Anderson, J. 2022 (accessed February) Facelina bostonensis, Scottish Nudibranchs www.nudibranch.org/Scottish%20Nudibranchs/facelina-boston…
Bergh, L. S. R. (1882). Beitrage zur Kenntniss der Aeolidiaden. VII. Verhandlungen der koniglich-kaiserlich Zoologisch-botanischen Gesellschaft in Wien (Abhandlungen). 32: 7-74, pls. 1-6 www.biodiversitylibrary.org/item/49918#page/135/mode/1up (Acanthopsole vicina Bergh 1882. pp 29 – 34 and dissection diagrams
Taf. II -16 www.biodiversitylibrary.org/item/49918#page/183/mode/1up
Taf III www.biodiversitylibrary.org/item/49918#page/185/mode/1up
Carmona, L. 2020. Investigating the amphiatlantic status of Facelina bostoniensis (Couthouy, 1838) (Nudibranchia: Aeolidida). Journal of Molluscan Studies 86: 64–71. doi:10.1093/mollus/eyz034 academic.oup.com/mollus/article/86/1/64/5766684
Couthouy, J. P. 1838. Descriptions of new species of Mollusca and shells, and remarks on several Polypii, found in Massachussets Bay. Boston Journal of Natural History. 2(1): 53-111 www.biodiversitylibrary.org/page/32266711#page/69/mode/1up
iNaturalist, map of observations suggested to be F. bostoniensis (accessed February 2022, click squares to see images) www.inaturalist.org/observations?taxon_id=461834
McKay, D.W. & Smith, S.M. 1979. Marine Mollusca of East Scotland. Royal Scottish Museum, Edinburgh.
Prkić J., Petani A., Iglić Đ. & Lanča L., 2018. Stražnjoškržnjaci Jadranskoga mora, Slikovni atlas i popis hrvatskih vrsta / Opisthobranchs of the Adriatic Sea, Photographic atlas and list of Croatian species. Ronilački klub Sveti Roko, Bibinje, pp. 464.
Thompson, T.E. & Brown, G.H. 1984. Biology of opisthobranch molluscs 2. London, Ray Society.
Current taxonomy: World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=139908
Glossary
amphiatlantic = Occurring on both sides of the Atlantic Ocean.
bilaminate = formed of two layers.
buccal mass = anterior of digestive system including a radula, odontophore and muscles.
cerata = (sing. ceras) lobes on dorsum of aeolids and some other seaslugs.
cnidaria = hydroids, jellyfish, sea anemones etc. which possess cnidocytes.
cnidocytes = explosive stinging cells of hydroids, jellyfish, sea anemones etc. en.wikipedia.org/wiki/Cnidocyte
cnidosac = storage capsule at tips of cerata of Aeolidiidae, but not Dotidae, for ingested unexploded cnidocytes.
digestive gland = organ in gastropods which acts like the liver and pancreas in mammals to absorb food.
distal = away from centre of body or from point of attachment.
hermaphrodite, simultaneous = individual acts as both male and female at the same time with similar partner(s).
lamellae = small plates on rhinophores.
metapodium = hind portion of the foot. (adj. metapodial).
oesophagus = tube from mouth to stomach.
pericardium = sac containing heart, often visible behind rhinophores in aeolid sea slugs.
propodium = anterior portion of gastropod foot.
propodial = at the front of the foot.
rhinophore = chemo-receptor tentacle; many sea slugs have a pair on top of the head.
subapical = just below the apex.