Click image to enlarge with full caption. Main text below slider.
Limapontia capitata (O. F. Müller, 1774)
Revised July 2021.
Current taxonomy; World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=140229
Synonyms: Fasciola capitata O. F. Müller, 1774; Pontolimax capitatus (O. F. Müller, 1774); Limapontia nigra G. Johnston, 1835.
GLOSSARY below.
Description
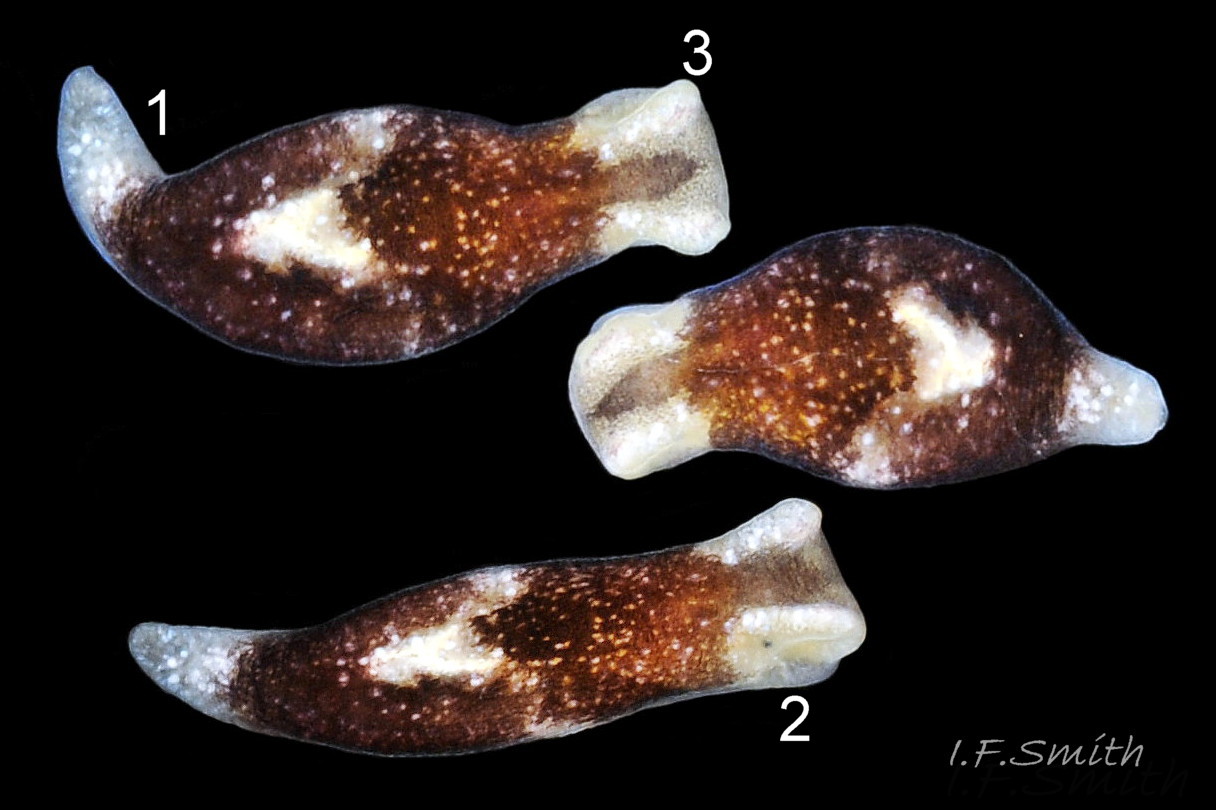
Usually up to 4 mm long, rarely 8 mm (Thompson, 1976). The smooth body has no tubercles, gills or appendages. It is dark brown ( 01 Limapontia capitata) or black ( 02 Limapontia capitata) except for the dorsal rim of the foot, metapodium, eye areas and head crests which are all translucent whitish, flecked with opaque white. There are often random light spots on the dark areas and, sometimes, small greenish patches and flecks. Usually, there is a large pale patch, often approximating to a heart shape, ( 03 Limapontia capitata) on the dorsum. Part of the patch is often translucent allowing sight of the heart beating within the translucent pericardium (Jensen, 1977).
The anus is a short distance behind and to the right of the midpoint of the body, but it is often difficult to see when it is not defecating.
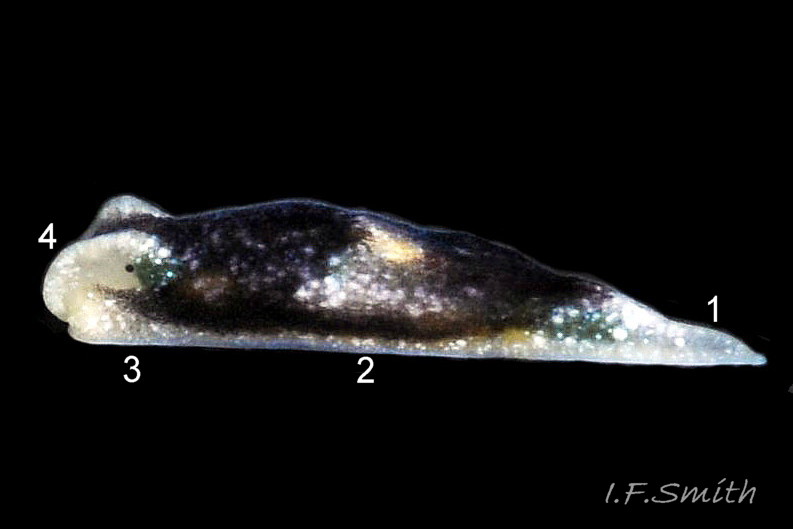
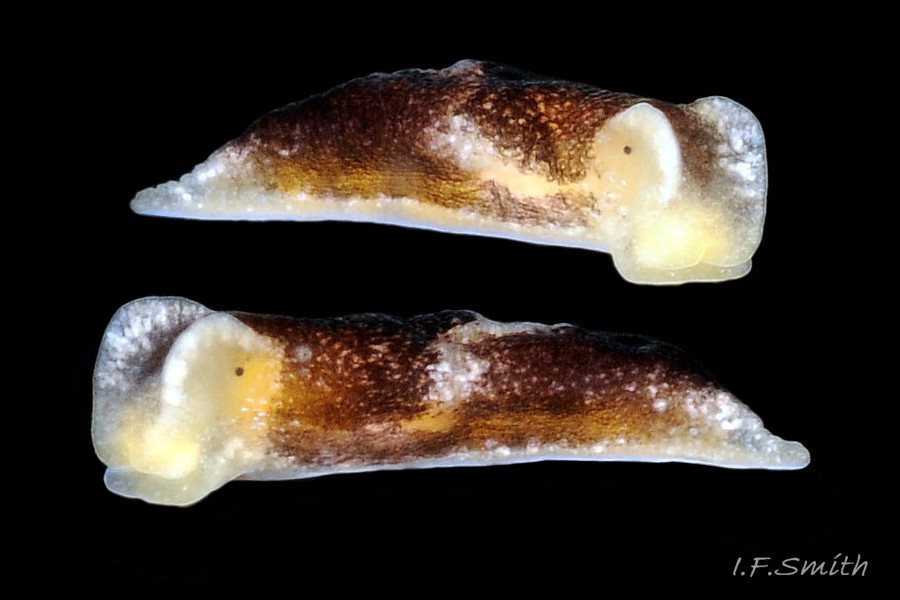
The head has a truncated anterior edge and usually extends beyond the foot ( 04 Limapontia capitata). There are no digitiform rhinophores but, above and in front of each eye, adults have a strong ( 05 Limapontia capitata) or weak ( 03 Limapontia capitata) head crest which is absent from some juveniles.
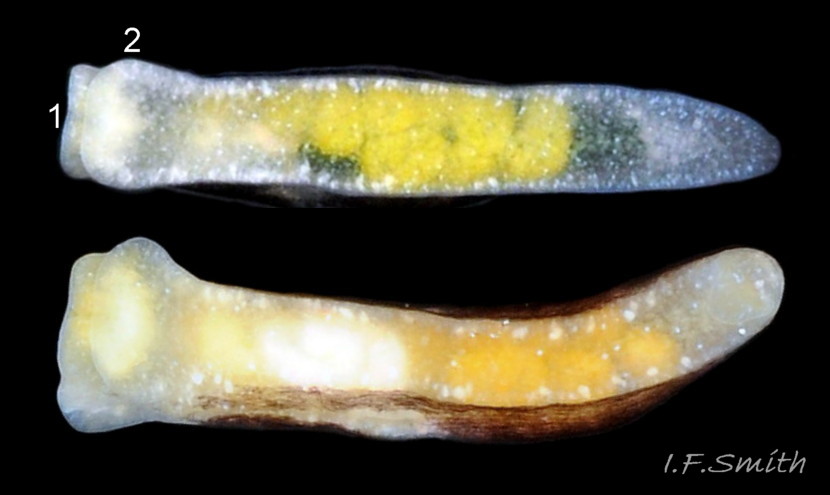
The foot has a translucent whitish sole spotted with white pigment. The yellow ovotestes of adults and/or green contents of the digestive gland may be visible through the sole ( 04 Limapontia capitata). The anterior of the foot is often slightly expanded but there are no propodial tentacles.
The substantial pale metapodium is c. 19-25% of the body length.
Key identification features
Limapontia capitata
1) Curved head crest above and in front of each eye ( 03 Limapontia capitata & 05 Limapontia capitata ), no ridge below eye. At some angles of view, crests can be mistaken for digitiform rhinophores ( 06 Limapontia capitata.
2) Substantial pale metapodium is c. 19-25% of body length.
3) Usually a large pale mark on the dorsum ( 03 Limapontia capitata).
4) Eye areas and head-crests whitish ( 02 Limapontia capitata)
5) Anus a short distance behind midpoint of body.
6) Sublittoral and all levels of the shore in pools and moist positions. Usually on Cladophora attached to hard substrate. Optimum salinity 30‰, can survive 5‰ to over 40‰, but sustainable population improbable below 10‰, the lower limit for spawning.
Similar species
Limapontia depressa Alder & Hancock, 1862 ( 07 Limapontia capitata)
1) No digitiform rhinophores but most have a raised rim around the pale eye patches which Alder & Hancock (1862) in their original species description refer to as ‘lateral crests’, and which Hancock clearly illustrated (item 4 on ). Most subsequent authors omit or deny the existence of the rim/crests on L. depressa (Barrett & Yonge, 1958; Gascoigne, 1975; Thompson, 1976; Hayward & Ryland, 1998; Kluijver et al.). Consequently, the rim is often mistaken for head crests of L. capitata. The rim varies in how much it is erected, being low when a specimen is not in good condition, and it may be difficult to discern in dorsal view of very dark specimens.
2) Pale metapodium (‘tail’) absent or negligible when viewed dorsally.
3) No large, pale, pigment mark on dorsum (occasionally a faded area).
4) Pale eye patches.
5) Dorsal anus close to posterior.
6) On tidal saltings in Britain in brackish or full marine salinity. Individuals adapt slowly and with difficulty to salinity change, but local populations are found adjusted to a wide range of salinities, to below 3‰ on the tidal River Dee, Wales. It lives sublittorally in the inner Baltic Sea, where the mean sea surface salinity is below 7‰ (Bendtsen et al.); a specimen near Helsinki, misidentified (when accessed in June 2021) as L. capitata, is at www.youtube.com/watch?v=6TBqOdGHmmI .
Limapontia senestra (Quatrefages, 1844) ( 08 Limapontia capitata)
1) Pair of digitiform rhinophores on head only when full grown. Earlier growth stages with rhinophores not fully developed can resemble head crests of L. capitata; rear in captivity when in doubt; rearing details in Smith (2014).
2) Pale metapodium is 13-18.5% of body length , smaller than on L. capitata but more noticeable than on L. depressa.
3) Often a small pale dorsal spot and lateral spots form a quincunx or similar; missing on translucent specimens with visible pale viscera which can be mistaken for the dorsal mark of L. capitata.
4) Eye patches and tentacles whitish.
5) Anus a short distance behind midpoint of body.
6) Full salinity, lagoons perhaps with salinity c. 20‰, and rock pools up to MHW on exposed coasts.
Habits and ecology
L. capitata tolerates a wide range of salinities; in the Kieler Bucht, Germany, 5‰ to 40‰ at 14°C, but spawning only occurs at over 10‰ (Seelemann, 1968 in Jensen 1977). The optimum salinity in the Kattegat, Denmark, for growth and spawning is 30‰ at 15°C, though spawn is abundant at over 15‰.
Coma occurs from heat at 38-40°C and from cold at about 1°C (Jensen, 1977). Formation of ice on a shore is usually accompanied by local temporary extinction of littoral L. capitata (Jensen, 1976).
It lives sublittorally and at all levels of the shore in pools and moist situations on its food algae, primarily Cladophora rupestris (09 Limapontia capitata & 10 Limapontia capitata ) but also Chaetomorpha linum, Bryopsis plumosa ( 11 Limapontia capitata ) and other Cladophora spp. (Jensen, 1975). These algal species are coenocytic with few or no internal cell walls subdividing the cytoplasm, which is consequently easily extracted by suction. Enteromorpha (currently genus Ulva) is sometimes mentioned as a food alga (Miller, 1962) but this is unlikely as all species in the order Ulvales, having uninucleate cells (Wichard et al. 2015), are not coenocytic, so unsuitable for suctorial feeding. Jensen (1975) observed a L. capitata grasping filaments of Enteromorpha in a feeding position, but it was unable to extract any cytoplasm. Cladophora. spp., Chaetomorpha linum and Bryopsis plumosa were equally favoured in experiments (Jensen, 1975), though in the wild most are found on Cladophora spp. as the other algae are less common. Individual L. capitata could change food in experiments, but were conservative, tending to remain on the first species encountered until all consumed. Cladophora glomerata, a freshwater species which grows well in the very low salinity of the inner Baltic (GBIF map) and forms large algal blooms in the Gulf of Finland (Berezina et al., 2007) was studied by A.-M. Jansson (1966, 1967 and 1970, in Jensen, 1975) on the island of Asko, south of Stockholm, but she found no L. capitata on it. However, L. depressa does feed on it and has been widely misidentified in the inner Baltic as L. capitata (misidentified L. depressa on probable C. glomerata at www.youtube.com/watch?v=6TBqOdGHmmI )
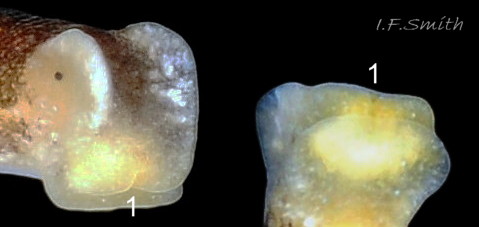
In L. capitata, the single row of radular teeth, adapted to only slitting and cutting ( 12 Limapontia capitata. ), confines it to suctorial feeding. The leading tooth is used to puncture algal cell walls whereas the newer, unused teeth function as a spear shaft. Recently worn out older teeth are retained in an ascus sac (Thompson, 1976). Further restrictions on which algal species can be utilized may be due to the chemical structure of the algal polysaccharides and to the algal filament diameters (Jensen, 1975).
The related L. depressa feeds by holding an algal filament vertically in the groove at the front of its head while it punctures it and sucks out the cytoplasm, leaving a colourless filament. There is an apparent upper limit on the diameter of filaments that can fit into the groove, as it was observed in captivity to exhaust all available narrow filaments but to leave the thicker ones unaffected (IFS pers. obs.). The groove in front of the mouth of L. capitata ( 13 Limapontia capitata ) is similar to that of L. depressa . The filaments of a coenocytic species of Trailiella were too thin for adult L. capitata to grip in their groove firmly enough for feeding (Jensen, 1975).
In 1973, at Hellebaek, Denmark, the intertidal population density of L. capitata peaked at 2370-2960 per litre of Cladophora in June, August and October just after settlement of newly metamorphosed juveniles less than 1.25 mm long from what seems to be three breeding events. The recorded population was zero in January to April, when water and air temperatures were below 10°C, and gradually increased in May, presumably originating from larval settlement from deeper water. The large population of L. capitata in summer was estimated to consume 1-10% of the total standing crop of Cladophora at Hellebaek (Jensen, 1975).
Copulation is by penetration by the stylet on the hypodermic penis into the body of the partner which lacks an aperture to receive it. The spawn mass, containing up to 800 ova, is deposited between June and November by two or more generations in Britain (Miller, 1962 in Thompson, 1976). The planktonic, veliger, larval stage lasts about a week at 16-17°C (Thompson, 1976). In Isefjord, Denmark, large numbers of planktonic veligers were recorded in April, August and December (Rasmussen, 1973), corresponding with hatching from the three spawning periods observed by Jensen (1975).
Distribution and status
L. capitata occurs from the Arctic to the Mediterranean and Black Sea. It extends into the Baltic to Rügen, Germany and the Øresund, Sweden further east than which the mean sea surface salinity (msss) is below 10‰ (Bendtsen et al, 2007). It may be over recorded because of incompletely developed rhinophores on juvenile L. senestra being mistaken for the crests of L. capitata. For details of misidentification and misrecording of Limapontia spp. in the inner Baltic, see the appendix below.
Common and widespread around Britain and Ireland. UK distribution map, NBN species.nbnatlas.org/species/NHMSYS0021056302 .
Appendix: Distribution of L. capitata in the Baltic Sea.
The first description of L. capitata, by Müller in 1774, was in the Baltic, ‘in Mari Balthico’. It is still present, sometimes abundantly (Jensen, 1975), in the outer Baltic to about 30° E. at Rügen, Germany (Schultze, 1849) and Øresund, Sweden. Working in the Kieler Bucht, Seelemann (1968 in Jensen 1977), found that the lower salinity limit for spawning is 10‰. The mean sea surface salinity (msss) of the inner Baltic east of Rügen-Øresund is below 10‰ (Bendtsen, 2007) and would be expected to prevent the establishment of sustainable populations of L. capitata. A study on the shores of Asko Island, south of Stockholm, (Jansson, 1966, 1967 and 1970, in Jensen, 1975), which conforms to expectations, found no L. capitata on Cladophora glomerata, a freshwater alga which grows well in the very low salinity of the inner Baltic and forms large algal blooms in the Gulf of Finland (Berezina et al., 2007).
Contrary to expectations, there are several reports of it east of Rügen-Øresund at,
1) Bornholm in 1863, current msss circa 7.5‰ (Meyer & Möbius, 1865–1872).
2) North of Stockholm at 61.1N, 17.2E, msss circa 5‰, in 1980 by Swedish Ocean Archive database (GBIF map, L. capitata).
3) Estonia, over 170 records, msss circa 5-6‰, 2008-2017 by Estonian Naturalists’ Society (GBIF map, L. capitata).
4) Finland, 450 records, msss circa 5-6‰, mainly 1990-2020, by Finnish Biodiversity Information Facility.
The Bornholm record has several reasons for reserve, apart from the low salinity. While the illustrations from Kieler Bucht ( 14 images of L. capitata in Meyer & Möbius. ) show that Meyer and Möbius (1865–1872) recognised correctly the features of L. capitata, the Bornholm specimens were found in 1863 when it is unlikely that M&M were familiar with Limapontia depressa, first described by Alder and Hancock only in the previous year and without published image. The specimens were collected for M&M by a fisherman who said that he found them abundantly under littoral stones. This is not the usual habitat of L. capitata, which lives on filamentous algae, mainly Cladophora spp.; one wonders how reliable the reported location is. It is desirable that this record be checked with fieldwork and photography.
The other localities have salinities well below the level suitable for spawning of L. capitata so its presence needs substantiation with detailed images. The only Baltic images labelled L. capitata found by IFS on the web are a video and two photographs from Finnish waters by K. Könönen at www.youtube.com/watch?v=6TBqOdGHmmI and laji.fi/en/taxon/MX.212476/images , which are all misidentified L. depressa lacking the substantial pale metapodium, large pale dorsal mark and distinct head crests of L. capitata [2024; images have been relabelled correctly as L. depressa . In 2012, on a blog by an artist for the Marine Research Centre at Stockholm University, there was a detailed painting labelled ’L. capitata’ from north of Stockholm, which was a perfect match for Hancock’s image of P. depressa ( 07 Limapontia capitata). See the ‘Key identification features’ and ‘Similar species’ sections of the main account above for detail of the historical confusion of the two spp.
Pruvot-Fol (1954) aggregated P. depressa with L. capitata as L. nigra as she could find no distinctive features to characterize them. In her description she used poor copies of 110 year old images of Limapontia spp. from Quatrefages (1844) and followed his omission of L. depressa which was not described by Alder and Hancock until 18 years after he wrote. Gascoigne (1975) and Thompson (1976) showed clear, anatomical differences which counter Pruvot-Fol’s opinion.
At the same time (June 2021) as showing multiple records of presumed L. capitata in Estonian and Finnish waters, the GBIF map for L. depressa and the Finnish Biodiversity Information Facility website have a complete absence in the same waters of records for L. depressa which has populations that can breed at the salinities found there, while L. capitata cannot. A video and two photographs from Finland and a painting from north of Stockholm, all mislabelled ‘L. capitata’, show that L. depressa does live in the inner Baltic. Over 30 records (1998-2012) on the GBIF map of L. depressa by the Swedish Ocean Archive database (SHARK) show that L. depressa lives on the coast of the inner Baltic in the Swedish counties of Kalmar and Blekinge.
Jonne Kotta of the Estonian Marine Institute, University of Tartu, agrees that all the Estonian records of L. capitata shown on GBIF are misidentified L. depressa and should be renamed on the database (J. Kotta, 2021, pers. comm., 14 June).
It is desirable that more photographs are obtained of Limapontia in the inner Baltic to substantiate or alter the evidence, reasoning and opinions presented above. This account will be amended if new evidence requires it.
Acknowledgements
I am most grateful to Kathe Jensen, Jonne Kotta and Vollrath Wiese for their help and advice with this account, but any errors or omissions are my (IFS) responsibility. I thank David Fenwick www.aphotomarine.com/index.html and Malcolm Storey www.bioimages.org.uk/ for use of their images.
References and links
Alder, J. and Hancock, A. 1862. Descriptions of a new genus and some new species of naked mollusc. Ann. mag. nat. hist. vol. 10, Third series, number LVIII: 261-265. [Original description of L. depressa on p. 264].
www.biodiversitylibrary.org/page/22162433#page/282/mode/1up
Barrett, J. and Yonge, C.M. 1958 Collins pocket guide to the sea shore. London, Collins.
Bendtsen, J., Söderkvist, J., Dahl, K., Hansen, J.L.S. and Reker, J. 2007. Model simulations of blue corridors in the Baltic Sea. BALANCE Interim Report No. 9.. Copenhagen. balance-eu.org/xpdf/balance-interim-report-no-9.pdf
Berezina, N. A., Tsiplenkina, I. G., Pankova, E. S. and Gubelit J. I. 2007. Dynamics of invertebrate communities in stony littoral of the Neva Estuary (Baltic Sea) under macroalgal blooms and bioinvasions. Transitional Waters Bulletin 1: 65-76. www.researchgate.net/publication/215447660_Dynamics_of_in…
Eliot, C.N.E. 1910. A monograph of the British nudibranchiate mollusca. London, Ray Society. Supplementary Volume. p. 141 [as L. nigra] archive.org/details/british_nudibranchiate_mollusca_pt8_l… (p. 151 of PDF).
Finnish Biodiversity Information Facility, Limapontia capitata overview page. laji.fi/en/taxon/MX.212476 images laji.fi/en/taxon/MX.212476/images [misidentified L. depressa]. Accessed 17 July 2021.
Gascoigne, T. 1975. A field guide to the British Limapontidae and Alderia modesta. J. Conch. Lond. 28: 359 – 364.
GBIF Distribution map of Limapontia capitata (O.F. Müller) Accessed 25 June, 2021. www.gbif.org/species/2298915
GBIF. Distribution map of Limapontia depressa Accessed 23 July, 2021. www.gbif.org/species/2298918
GBIF Distribution map of Cladophora glomerata (L.) Kütz. Accessed 14 June, 2021. www.gbif.org/species/5272770
Hayward, P.J. & Ryland, J.S. 1996. Handbook of the marine fauna of North-west Europe. Oxford, Oxford University Press.
Jeffreys, J. G. 1869. British conchology. vol. 5 (1869). London, van Voorst. [As L. nigra] archive.org/details/britishconcholog05jeffr/page/28/mode/1up
Jensen, K. R. 1975. Food preference and food consumption in relation to growth of Limapontia capitata (Opisthobranchia, Sacoglossa). Ophelia 14(1-2): 1-14. abstract
www.tandfonline.com/doi/abs/10.1080/00785236.1975.10421967
Jensen, K. R. 1976. The importance of Limapontia capitata (Mueller) (Opisthobranchia, Sacoglossa) as a primary consumer in the Cladophora-belt. 10th Europ. Symp. mar. Biol. 2: 339-350.
Jensen, K. R. 1977. Optimal salinity and temperature intervals of Limapontia capitata (Opisthobranchia, Sacoglossa) determined by growth and heart rate measurements. Ophelia, 16 (2): 175 – 185.
Kluijver, M.J. de, Ingalsuo S.S. & Bruyne, R.H. de. Mollusca of the North Sea, Limapontia depressa. Marine Species Identification Portal. (accessed 20 June 2021) species-identification.org/species.php?species_group=moll…
Meyer, H. A. & Möbius, K. 1865 – 1872. Fauna der Kieler Bucht. Band 1: Die Hinterkiemer oder Opisthobranchia. Leipzig, W. Engelmann. [As Pontolimax capitatus]
www.biodiversitylibrary.org/item/47329#page/57/mode/1up [images]
www.biodiversitylibrary.org/item/47329#page/55/mode/1up [text]
Miller, M.C. 1962. Annual cycles of some Manx nudibranchs, with a discussion of the problem of the migration. J. Anim. Ecol. 31(3): 545-569 www.jstor.org/stable/2053?seq=1
Müller, O. F. 1774. Vermium terrestrium et fluviatilium, seu animalium infusoriorum, helminthicorum, et testaceorum, non marinorum, succincta historia. Vol. 1, Pars Altera: p. 70. [1774]. Havniæ (Copenhagen) & Lipsiæ (Leipzig), Heineck & Faber. [original description as Fasciola capitata] www.biodiversitylibrary.org/item/50344#page/236/mode/1up
Pruvot-Fol, A. 1954. Faune de France. Mollusques opisthobranches. Paris, P. Lechevalier. faunedefrance.org/bibliotheque/docs/A.PRUVOT-FOL(FdeFr58)Mollusques.pdf
Quatrefages J.L.A. de. 1844. Sur les Gastéropodes Phlébentérés (Phlebenterata Nob.), ordre nouveau de la classe des Gastéropodes, proposé d’après l’examen anatomique et physiologique des genres Zéphyrine (Zephyrina Nob.), Actéon (Acteon Oken), Actéonie (Acteoniæ Nob.), Amphorine (Amphorina Nob.), Pavois (Pelta Nob.), Chalide (Chalidis Nob.). Annales des Sciences Naturelles. ser. 3, 1: 129-183, pls 3-6. biodiversitylibrary.org/page/13407269
Rasmussen, E. 1973. Systematics and ecology of the Isefjord marine fauna (Denmark). Ophelia, 11, 1-495.
Schultze, M.S. 1849. Ueber die Entwickelung des Tergipes lacinulatus. Archiv für Naturgeschicht. 15: 270. www.biodiversitylibrary.org/item/48696#page/670/mode/1up
Seelemann, U. 1968. Zur Überwindung der biologischen Grenze Meer-Land durch Mollusken. II. Untersuchungen an Limaponita capitata, Limapontia depressa und Assiminea grayana. Oekologia. 1: 356-368 www.jstor.org/stable/4214499
Smith, I.F. 2014. Rearing and breeding the sacoglossan sea slug, Limapontia senestra (Quatrefages, 1844). Mollusc World 34: 16-18. Conchological Society of Great Britain and Ireland. www.researchgate.net/publication/352982521_Limapontia_sen…
Thompson, T.E. 1976. Biology of opisthobranch molluscs 1. London, Ray Society.
Wichard, T., Charrier, B., Mineur, F., Bothwell, J. H., De Clerck, O. and Coates, J. C. 2015. The green seaweed Ulva: a model system to study morphogenesis. Frontiers in plant science. www.frontiersin.org/articles/10.3389/fpls.2015.00072/full
Current taxonomy; World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=140229
GLOSSARY
coenocytic = (of algae) with parts made up of multinucleate, large masses of cytoplasm enclosed by the wall of each large cell.
cytoplasm = gelatinous liquid that fills the inside of a cell; ‘cell sap’.
digitiform = shaped like a finger.
dorsum = upper outer surface of an organism.
metapodium = hind part of the foot.
MHW = mean high water level.
multinucleate = (of cells) having more than one nucleus per cell, i.e., multiple nuclei share one common cytoplasm.
ovotestes = (plural) hermaphrodite organs serving as both ovary and testes.
pericardium = sac containing the heart.
plankton = animals and plants that drift in pelagic zone (main body of water).
polysaccharides = (in algae) molecular structural components of cell walls.
propodial = (adj.) at the front of the foot.
radula = usually a chitinous ribbon with rows of teeth to rasp food, but on Sacoglossa a line of single, fused teeth used like a scalpel to pierce algal cells.
quincunx = pattern of five as on dominoes or dice.
radular = of the radula.
rhinophore = chemo-receptor tentacle; nudibranch and most sacoglossan sea slugs have a pair on top of the head.
salting = salt tolerant vascular vegetation at MHW to EHWS; preferred synonym for “saltmarsh” as much of a salting is not marshy.
siphonaceous = (of algae) entire thallus (‘plant’) is coenocytic with no internal cell walls subdividing the cytoplasm.
stylet = hard, sharp, slender piercing structure.
suctorial = (adj.) sucking
uninucleate = (of cells) having one nucleus per cell.
veliger = shelled larva of marine gastropod or bivalve mollusc which moves by action cilia on a velum (bilobed flap).