Click image to enlarge with full caption. Main text below slider.
Limapontia depressa Alder & Hancock, 1862
PDF available at www.researchgate.net/publication/353270734_Limapontia_dep…
Current Taxonomy: World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=140230
Synonyms: Limapontia depressa olivaria Gascoigne, 1978.
GLOSSARY below.
Description
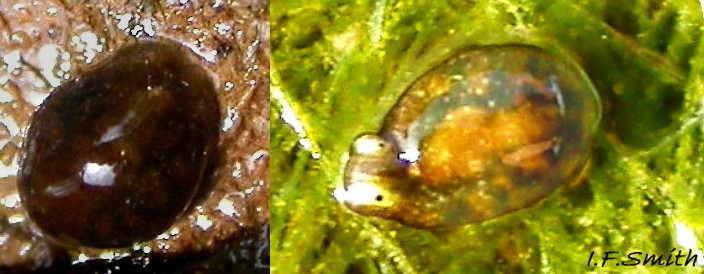
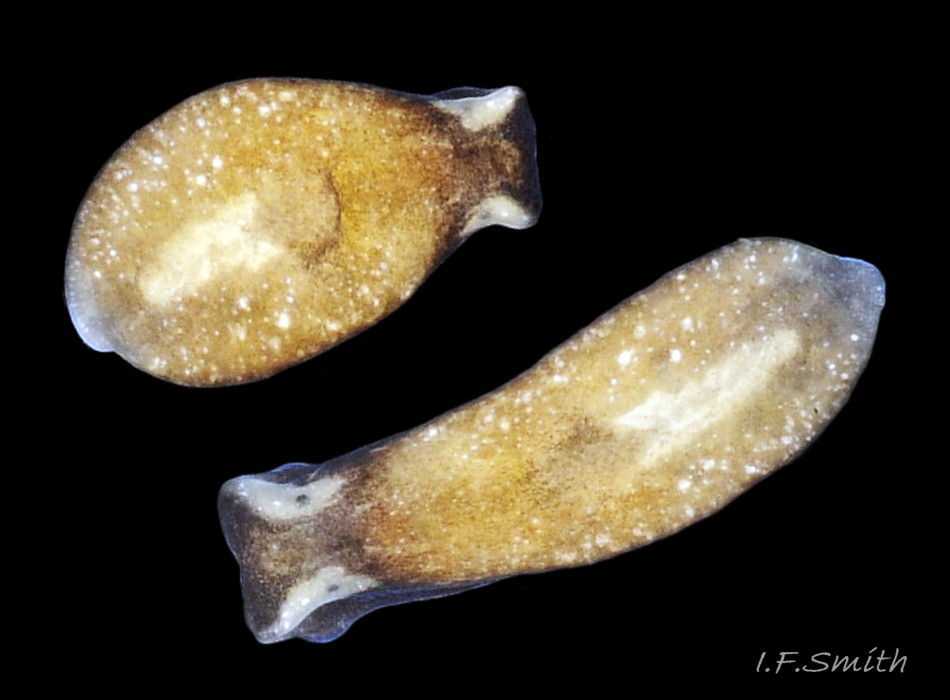
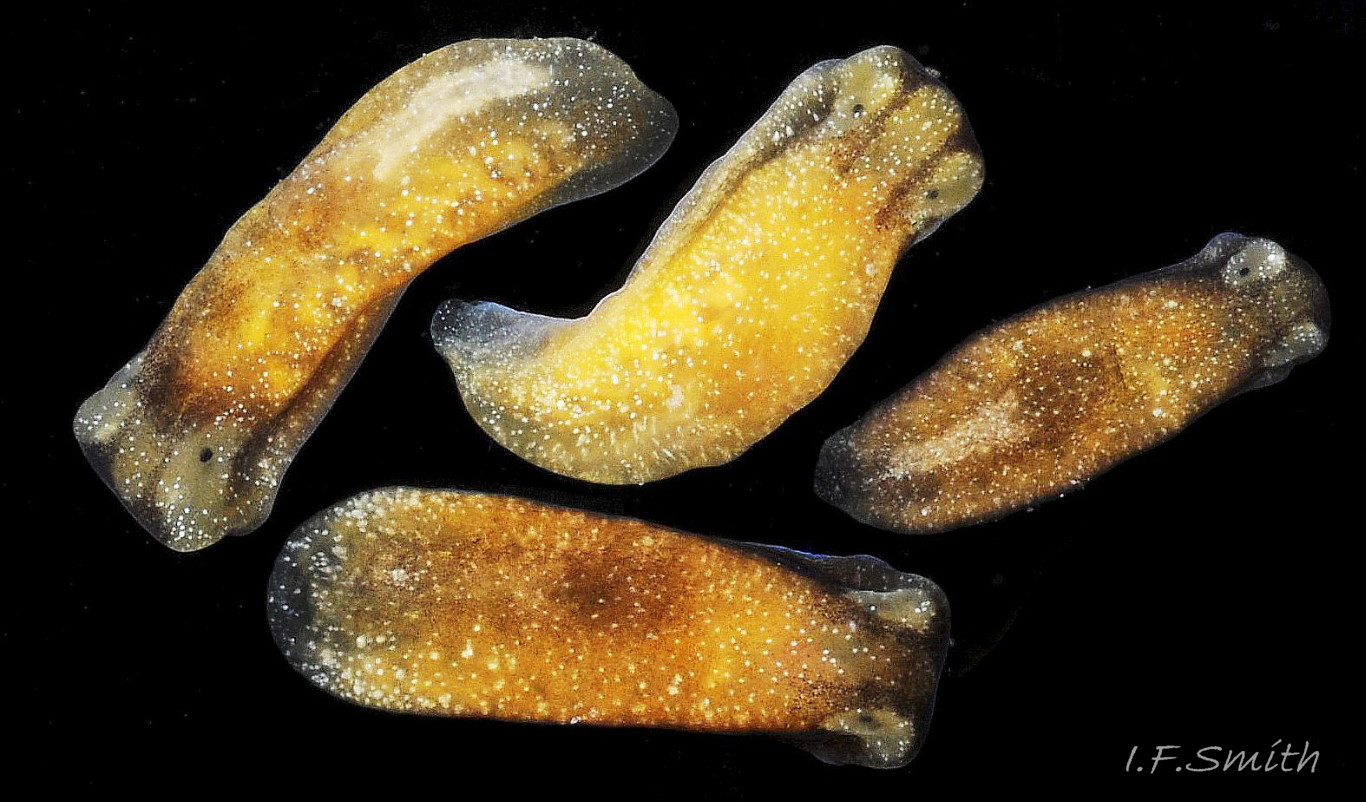
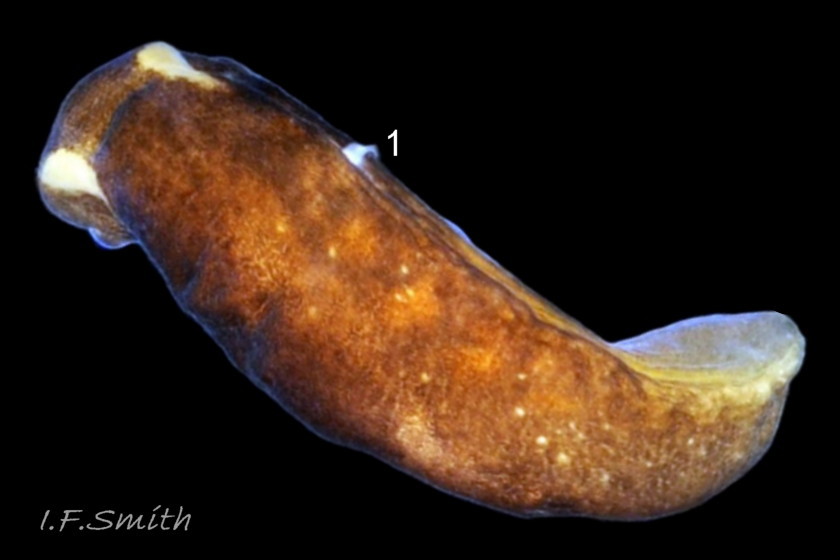
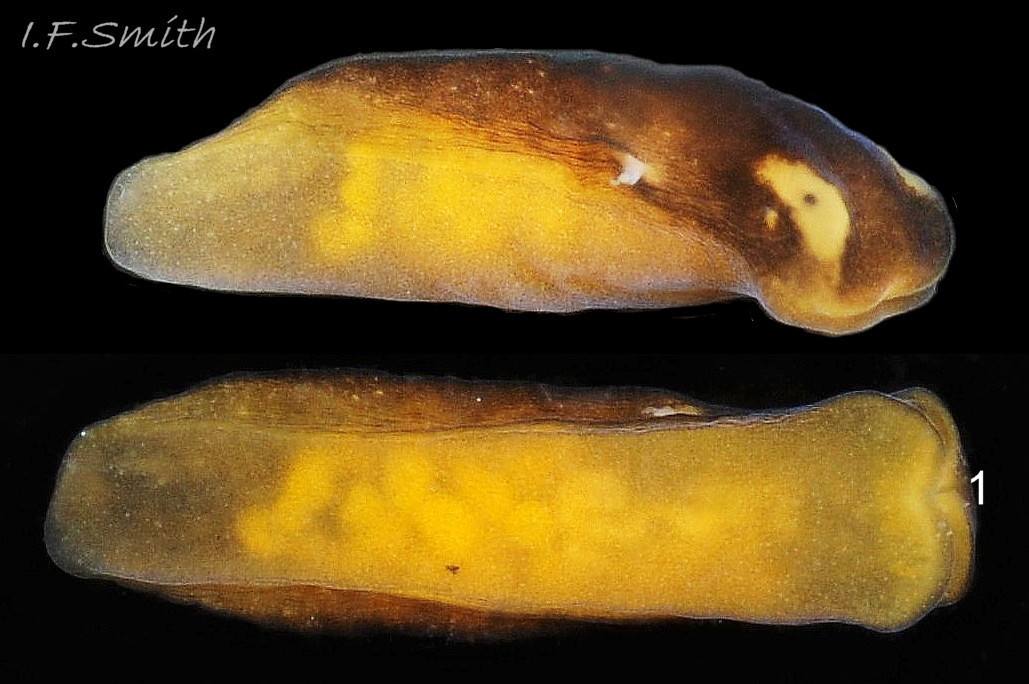
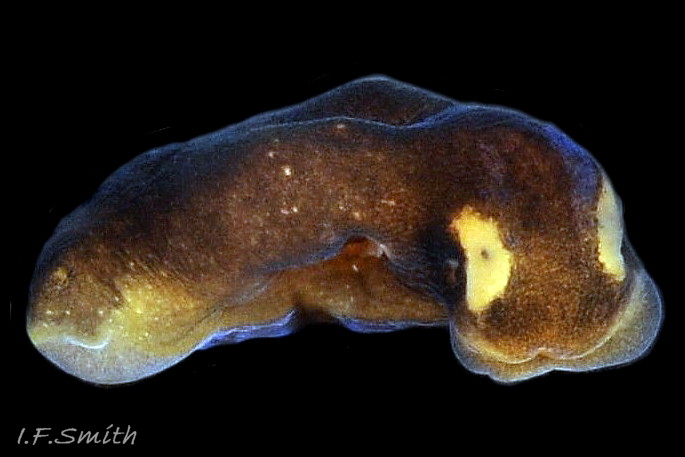
The maximum length of L. depressa is about 6 mm. When extended, it has a broad oblong-ovate outline ( 01 Limapontia depressa. ), and it can contract to a mucus-coated spheroid ( 02 Limapontia depressa. The usual colour is some shade of brown, varying from yellowish to black, usually with small, random, paler marks ( 01 Limapontia depressa. ). Most lack any large pale marks, but occasionally there is a faded dorsal area ( 03 Limapontia depressa ), and some are translucent showing the branched digestive gland which varies from dark green to yellowish, depending on diet ( 04 Limapontia depressa ). Colour varies with diet and habitat; individuals from the same site are often similar ( 05 Limapontia depressa ).
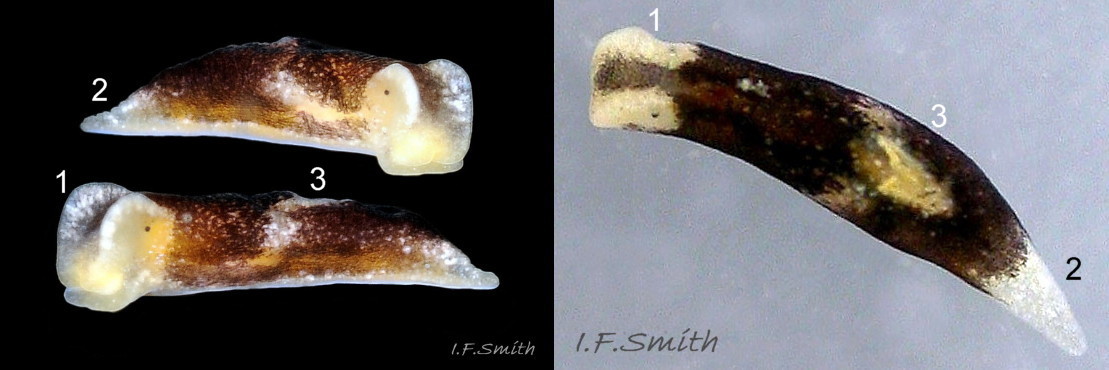
The position of the bursa copulatrix is sometimes indicated by a pale patch on the right-hand side, about a quarter of the body length from anterior (06 Limapontia depressa). The white penis has a sharp, curved stylet at the tip ( 07 Limapontia & 09 Limapontia depressa). The anus is near the posterior of the body, but unobtrusive except during excretion of grey-white semi-liquid faeces ( 08 Limapontia depressa.
The head is widest at its truncated anterior; its rounded corners are about 90° ( 03 Limapontia depressa). Each black eye is in a pale eye patch which may be saucer-like with distinct raised rim ( 01 Limapontia depressa. , 03 Limapontia depressa & 05 Limapontia depressa) or with indiscernibly raised edges ( 08 Limapontia depressa). The anterior of the head has a vertical groove ( 09 Limapontia depressa) leading to the mouth, which is used for holding a filament of alga vertically when feeding. The sole of the foot is translucent and paler than the dorsum. It often shows the yellow, spheroid ovotestes ( 09 Limapontia depressa) and colours of the viscera and content of the digestive gland. The foot is widest at the slightly concave, truncate anterior. Dorsally, there is no pale metapodium visible, but a negligible one may be seen if the sole is slightly twisted into view (item 3 on 01 Limapontia depressa. ).
Key identification features
Limapontia depressa
1) No digitiform tentacles, but most have a raised rim around the pale eye patches ( 01 Limapontia depressa. ) which Alder & Hancock (1862) in their original species description refer to as ‘lateral crests’, and which Hancock clearly illustrated (item 4 on 01 Limapontia depressa. ). Most subsequent authors omit or deny the existence of the rim/crests on L. depressa (Barrett & Yonge, 1958; Gascoigne, 1975; Thompson, 1976; Hayward & Ryland, 1998; Kluijver et al.). Consequently, the rim is often mistaken for head crests of L. capitata. The rim varies in how much it is erected, being low when a specimen is not in good condition, and it may be difficult to discern in dorsal view of very dark specimens. See appendix in Smith (2021) for details of historical misidentification of L. depressa as L. capitata.
2) Pale metapodium (‘tail’) absent or negligible when viewed dorsally ( 01 Limapontia depressa. )
3) Usually, no large pale mark on dorsum ( 01 Limapontia depressa. ); occasionally a faded patch ( 03 Limapontia depressa).
4) Pale eye patches.
5) Dorsal anus close to posterior ( 08 Limapontia depressa).
6) On tidal saltings in Britain in brackish or full marine salinity. Individuals adapt slowly and with difficulty to salinity change, but local populations are found adjusted to a wide range of salinities, to below 3‰ on the tidal River Dee, Wales. Sublittoral in inner Baltic Sea where mean sea surface salinity below 7‰ (Bendtsen et al.); specimen near Helsinki, misidentified (when accessed in June 2021) as L. capitata, at www.youtube.com/watch?v=6TBqOdGHmmI .
Similar species
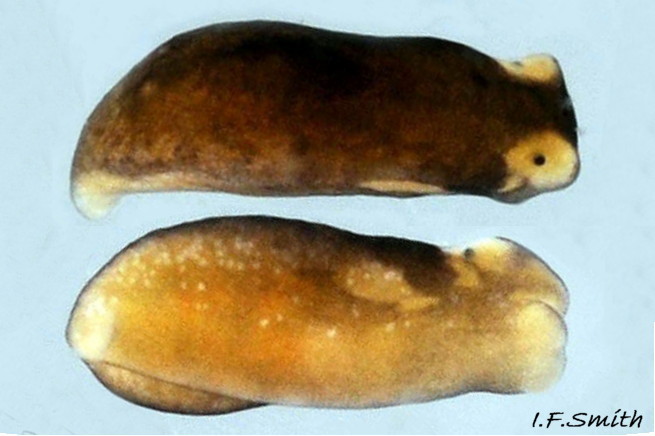
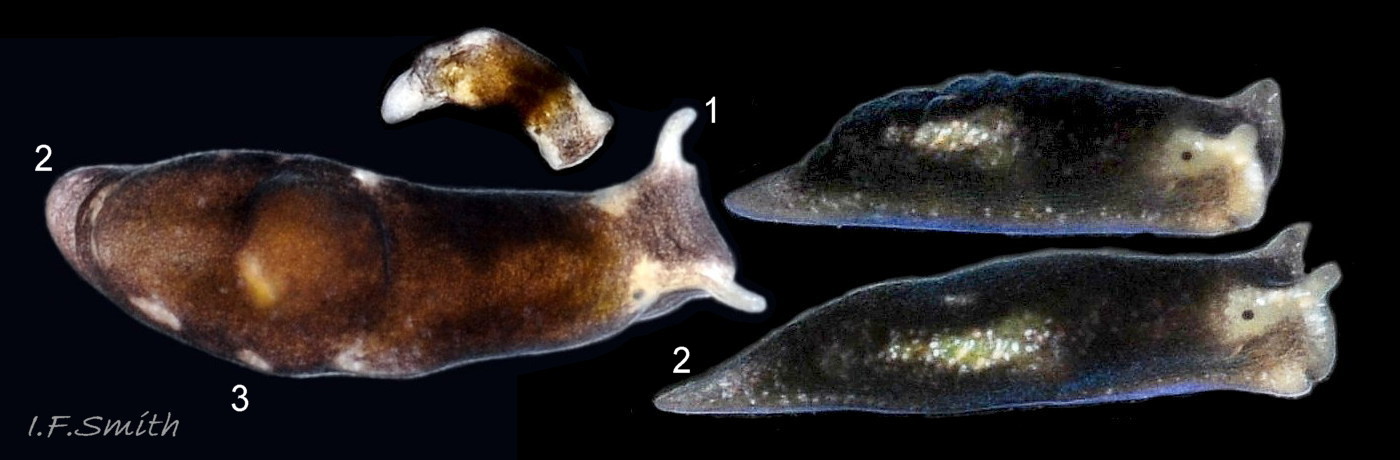
Limapontia capitata (O. F. Müller, 1774) ( 10 Limapontia depressa)
1) Curved head crest above and in front of each eye, no ridge below eye. Juveniles with weakly developed head crests can be mistaken for L. depressa; rear in captivity if in doubt.
2) Substantial whitish metapodium (‘tail’).
3) Usually a large pale mark on the dorsum.
4) Eye areas and head-crests whitish.
5) Anus a short distance behind midpoint of body.
6) Sublittoral and all levels of the shore in pools and moist positions. Usually on Cladophora attached to hard substrate. Optimum salinity 30‰, can survive 5 to over 40‰, but sustainable population improbable below 10‰, the lower limit for spawning.
Limapontia senestra (Quatrefages, 1844) ( 11 Limapontia depressa)
1) Pair of digitiform rhinophores on head only when full grown. Earlier growth stages with rhinophores not fully developed can be mistaken for L. depressa or L. capitata; rear in captivity if in doubt.
2) Pale metapodium, smaller than on L. capitata but more noticeable than on L. depressa.
3) Often a small pale dorsal spot and some pale lateral spots.
4) Eye patches and tentacles whitish.
5) Anus a short distance behind midpoint of body.
6) Full salinity, lagoons perhaps with salinity c. 20‰, and rock pools up to MHW on exposed coasts.
Habits and ecology
In Britain and Ireland, L. depressa is found at mid- and upper-tide levels of saltings and tidal rivers; those at EHWS are almost terrestrial. It is sublittoral in the almost tideless inner Baltic. As a species, it is extremely euryhaline; some populations living where water at high tide is fully marine, >30‰ ( 12 Limapontia depressa), and others where inundating water is < 3‰ ( 13 Limapontia depressa). But individuals are stenohaline, contorting ( 14 Limapontia depressa) and climbing out if placed in water of salinity differing from what they are accustomed to.
It lives on Vaucheria and other filamentous algae ( 12 Limapontia depressa), and on bare mud, often where little or no alga is visible, in salting ditches (fig 15 15 Limapontia depressa), on tidal river banks ( 16 Limapontia depressa, submerged in shallow pools ( 17 Limapontia depressa), and around edges of tidal pools on grazed saltings ( 13 Limapontia depressa). When dry or threatened, it contracts into a spheroid and exudes mucus ( 02 Limapontia depressa). If desiccation or uncongenial salinity threaten, it conceals itself just under the surface of mud ( 15 Limapontia depressa).
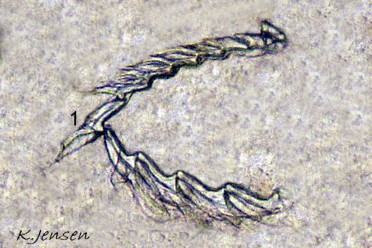
L. depressa feeds on coenocytic, filamentous algae, holding a filament vertically in a groove at the front of its head while it punctures it and sucks out the cytoplasm, leaving a colourless tube ( 18 Limapontia depressa. ). Its single row of radular teeth, adapted to only slitting and cutting ( 19 Limapontia depressa. ), confines L. depressa to suctorial feeding. The leading tooth is used to puncture algal cell walls whereas the newer, unused teeth function as a spear shaft. All worn out older teeth are retained in an ascus sac (Thompson, 1976).
Vaucheria sp. is usually quoted as its food, but probably other coenocytic, filamentous species with few or no wall divisions are also used in mats of mixed species. There is apparently an upper diameter limit on filaments that can fit into the feeding groove at the front of the head, as it was observed in captivity to exhaust all available narrow filaments but to leave the thicker ones unaffected (IFS pers. obs.). Specimens on bare mud or muddy sand probably feed on microscopic organisms, possibly diatoms, coating the substrate, which can be sucked in without any cutting required ( 20 Limapontia depressa ).
L. depressa is a simultaneous hermaphrodite. Copulation is by mutual hypodermic impregnation. There is no female opening to accept the penis. The sharp style on the end of the penis (figs. 7 07 Limapontia depressa & 9 09 Limapontia depressa) punctures the body wall of its mate to enable injection of sperm. Spawn ( 18 Limapontia depressa. ) is laid on filamentous alga, when available, from May-September in Britain. The ova are much smaller than L. senestra eggs, but more numerous; up to about 200 per spawn mass. Veliger larvae then live in the plankton for up to four weeks before metamorphosis. Both species can be reared in captivity; details in Smith (2014).
Distribution and status
L. depressa occurs from Norway to the Bassin d’Arcachon in the Bay of Biscay (Thompson, 1976), including the Baltic probably as far as Vaasa, Finland, in the Gulf of Bothnia. GBIF distribution map (incomplete) www.gbif.org/species/2298918 . Details of its mistaken recording as L. capitata in the inner Baltic are given in an appendix in Smith (2021). It is widespread and locally abundant around Britain and Ireland on salt marshes, tidal rivers and estuaries; also in the shallow sublittoral of the inner Baltic. It is probably over looked in many suitable places because of its small size, drab colour and habit of burying itself in the surface layer of mud.NBN, UK distribution map species.nbnatlas.org/species/NBNSYS0000176144 .
Acknowledgements
I thank Kathe Jensen, Jonne Kotta and Vollrath Wiese for their help concerning Limapontia species. Any errors or omissions are my (IFS) responsibility.
References and links
Barrett, J. and Yonge, C.M. 1958 Collins pocket guide to the sea shore. London, Collins.
Bendtsen, J., Söderkvist, J., Dahl, K., Hansen, J.L.S. and Reker, J. 2007. Model simulations of blue corridors in the Baltic Sea. BALANCE Interim Report No. 9.. Copenhagen. balance-eu.org/xpdf/balance-interim-report-no-9.pdf
Eliot, C.N.E. 1910. A monograph of the British nudibranchiate mollusca. London, Ray Society. Supplementary Volume.
Gascoigne, T. 1975. A field guide to the British Limapontidae and Alderia modesta. J. Conch. Lond. 28: 359 – 364.
Hayward, P.J. & Ryland, J.S. 1996. Handbook of the marine fauna of North-west Europe.
Jeffreys, J. G. 1869. British conchology. vol. 5 (1869). London, van Voorst. archive.org/details/britishconcholog05jeffr/page/28/mode/1up
Kluijver, M.J. de, Ingalsuo S.S. & Bruyne, R.H. de. Mollusca of the North Sea, Limapontia depressa. Marine Species Identification Portal. (accessed 11 July 2021.)
www.species-identification.org/species.php?species_group=…
McMillan, N.F. 1946. Limapontia depressa in North Wales. J. Conch. Lond. 22: 220.
Smith, I.F. 2014. Rearing and breeding the sacoglossan sea slug, Limapontia senestra (Quatrefages, 1844). Mollusc World 34: 16-18. Conchological Society of Great Britain and Ireland. www.researchgate.net/publication/352982521_Limapontia_sen…
Smith, I.F. 2021. Limapontia capitata (O. F. Müller, 1774) Identification and Biology flic.kr/s/aHsmVLYE4o
Thompson, T.E. 1976. Biology of opisthobranch molluscs 1. London, Ray Society.
Current Taxonomy: World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=140230
GLOSSARY
bursa copulatrix = (a.k.a. spermatheca) copulatory pouch or sac for receiving sperm.
coenocytic = (of algae) with parts made up of multinucleate, large masses of cytoplasm enclosed by the wall of each large cell.
diatom = microscopic aquatic alga with siliceous cell-walls.
digitiform = shaped like a finger.
euryhaline = able to tolerate a wide variation in salinity.
EHWS = extreme high water spring tide (usually near equinoxes).
hermaphrodite, simultaneous = individual acts as both male and female at the same time with similar partner(s).
plankton = animals and plants that drift in the pelagic zone (main body of water).
metapodium = hind part of the foot; ‘tail’.
ovotestis = (pl. ovotestes) hermaphrodite organ serving as both ovary and testis.
radula = usually a chitinous ribbon with rows of teeth to rasp food, but on Sacoglossa a line of single, fused teeth used like a scalpel to pierce algal cells.
radular = of the radula.
rhinophore = chemo-receptor tentacle; many sea slugs have a pair on top of the head.
salting = area of salt tolerant vascular plants rooted in sediment between mean high water mark (MHW) and extreme high water of spring tides (EHWS). [preferred synonym for “saltmarsh” as much of a salting is not marshy].
stenohaline = unable to tolerate a wide variation in salinity.
veliger = shelled larva of marine gastropod or bivalve mollusc which moves by action cilia on a velum (bilobed flap). Stage may be passed in plankton or within liquid-filled egg-capsule.