Click image to enlarge with full caption. Main text below slider.
Limapontia senestra (Quatrefages, 1844).
PDF available at www.researchgate.net/publication/353218914_Limapontia_sen…
Current taxonomy; World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=140231
Synonyms: Actaeonia senestra Quatrefages, 1844; Actaeonia corrugata Alder & Hancock, 1848; Cenia cocksi Alder & Hancock, 1848.
GLOSSARY below.
Description
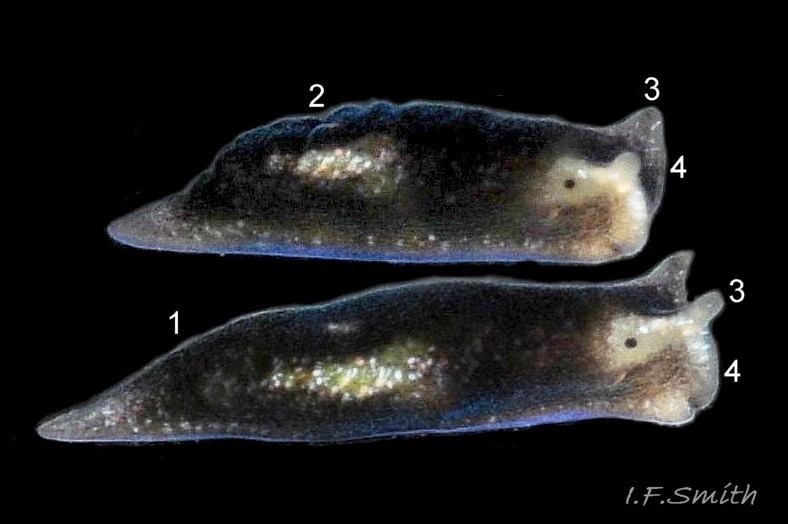
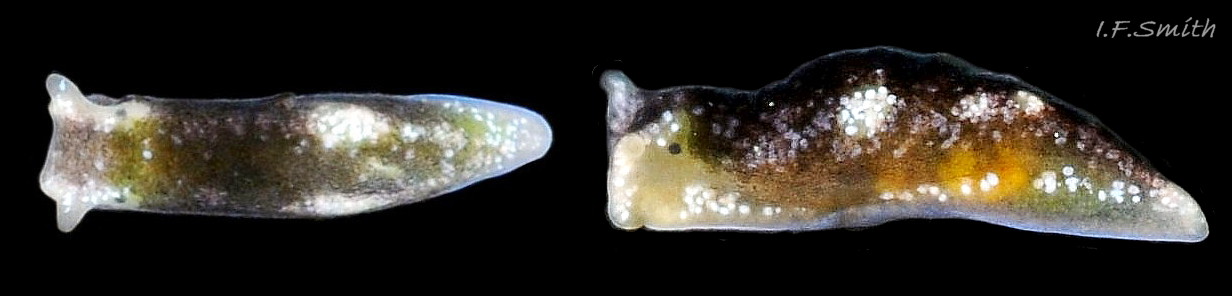
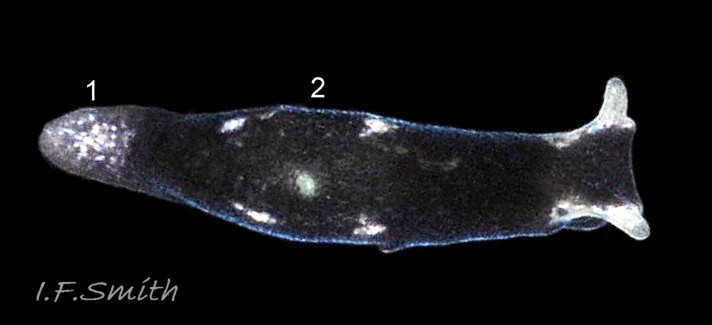
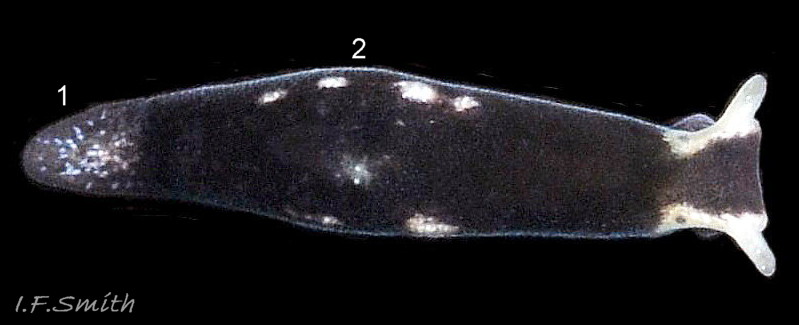
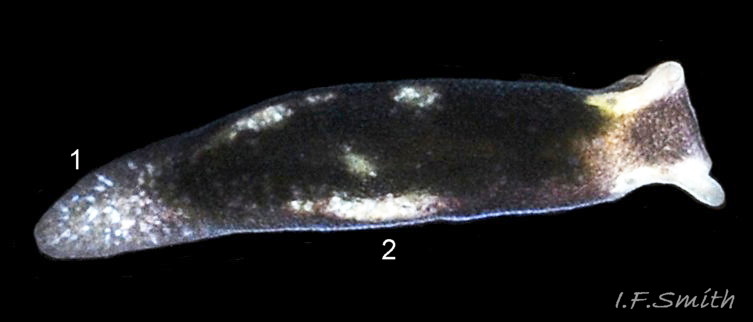
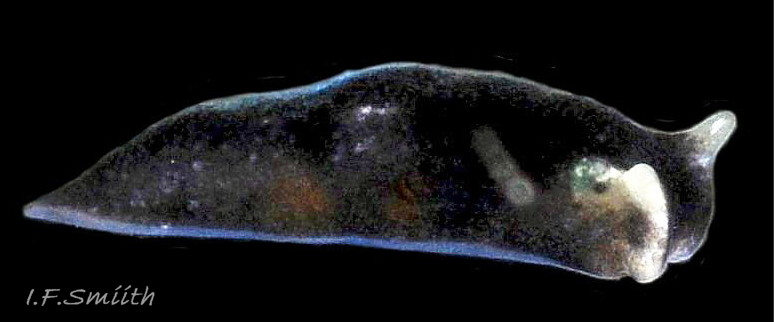
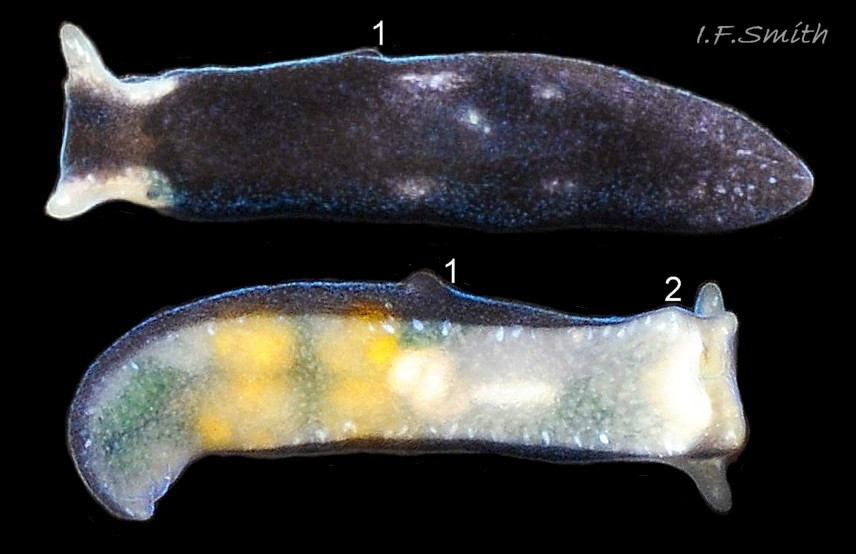
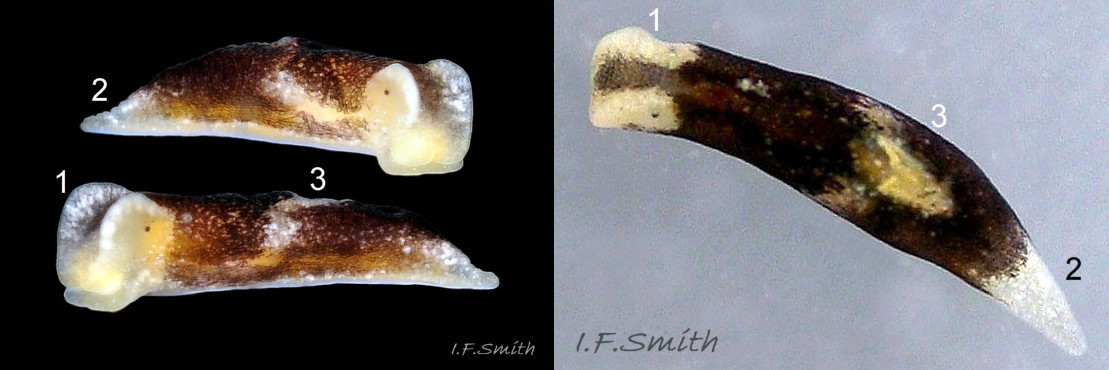
L. senestra grows up to 6 mm long. The smooth body has no tubercles but is occasionally wrinkled, which gave rise to the synonymous A. corrugata of A&H, 1848 ( 01 Limapontia senestra. ). The body is black ( 01 Limapontia senestra. ) or brown ( 02 Limapontia senestra ) and occasionally translucent, showing the colours of the internal organs ( 03 Limapontia senestra & 24 Limapontia senestra ). There is often a small, pale greyish or yellowish mark on the dorsum and two ( 04 Limapontia senestra. ) or more marks ( 05 Limapontia senestra. ), sometimes merging into a line ( 06 Limapontia senestra ), on each flank, or the marks may be obscure/absent ( 07 Limapontia senestra ). Quincunx pattern of five dots is regarded as typical ( 04 Limapontia senestra. ). There may also be scattered opaque white flecks, especially on the protruding metapodium (tail) ( 04 Limapontia senestra. ) and edge of the foot ( 03 Limapontia senestra ).
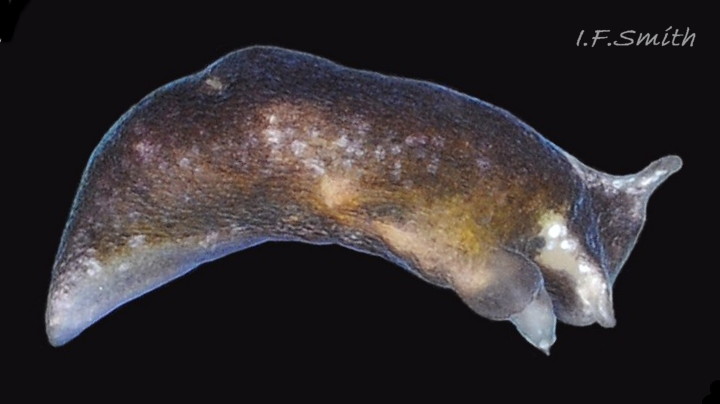
The anus is a short distance behind the mid-point of the body, but is difficult to see when not defecating, unless the specimen is translucent ( 24 Limapontia senestra ). The yellow-orange ovotestes and dark green digestive gland can be seen through the translucent foot ( 08 Limapontia senestra ) or occasionally translucent body ( 24 Limapontia senestra ). The penis is white with a sharp, curved stylet at the tip ( 09 Limapontia senestra . ). There is a small swelling over the bursa copulatrix ( 10 Limapontia senestra ).
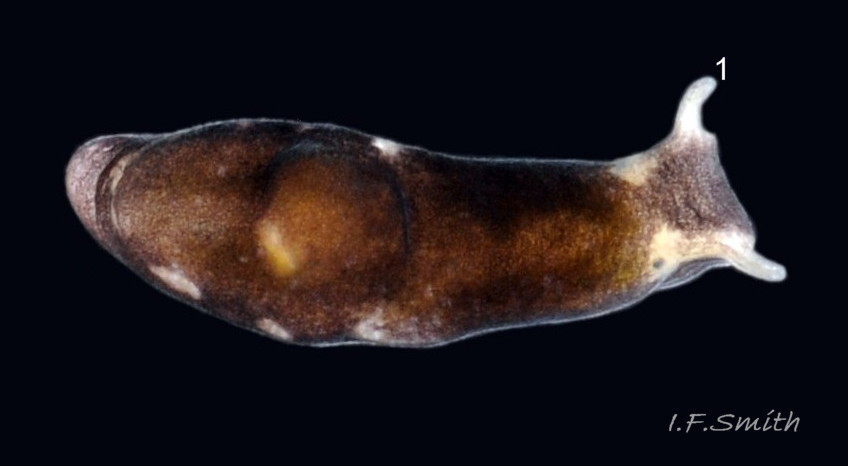
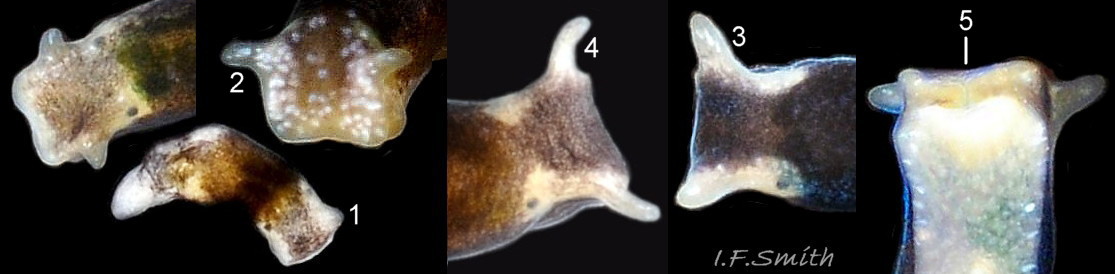
The head can extend forwards to almost horizontal, or contract to vertical ( 11 Limapontia senestra ). It has a slit at the anterior for holding filaments of alga while feeding ( 12 Limapontia senestra ). There is a medial band of black or brown, varying in intensity with extension. The rhinophores and eye patches are translucent whitish with varying amounts of opaque white flecking ( 12 Limapontia senestra ). When more than 2 mm long, L. senestra has spatulate rhinophores ( 01 Limapontia senestra. ). They become cylindrical, digitiform only when fully developed ( 13 Limapontia senestra ); size when this is achieved varies between individuals. “Ridges run forwards from the rhinophores to the front of the head” (Eliot, 1910). They are shown on Eliot’s image and more prominently in the original species description by Quatrefages (1844) who called them thick ridges, “crêtes épaisses” (15 Limapontia senestra). (Roginskaya, 2000, opined that Quatrefages’ image was of an abnormal specimen). The ridges are usually more prominent on young adults ( 01 Limapontia senestra. ) than those with fully developed digitiform rhinophores ( 02 Limapontia senestra & 16 Limapontia senestra). The ridge prominence varies on individuals and is often slight or absent when an animal is not fully extended ( 11 Limapontia senestra ). Rhinophores start to develop when L. senestra is about 1.5 mm long ( 12 Limapontia senestra & 03 Limapontia senestra ). Smaller early juveniles have low ridges but no rhinophores ( 14 Limapontia senestra ) and are easily mistaken for L. capitata or L. depressa.
The anterior of the foot is concave centrally and slightly swollen laterally but has no propodial tentacles or extensions ( 10 Limapontia senestra ). Apart from opaque white flecks around the periphery, the sole is translucent showing the colours of viscera ( 08 Limapontia senestra ). The extended pale metapodium is about 13-18.5% of the fully extended slug’s length ( 04 Limapontia senestra. ); shorter than on L. capitata (c. 19-25%) and longer than the negligible one on L. depressa.
Key identification features
Limapontia senestra
1) Spatulate rhinophores on head when 2 mm long or larger, with or without anterior ridge, becoming cylindrical digitiform when fully developed ( 13 Limapontia senestra ). Juveniles have only ridges above the eyes ( 14 Limapontia senestra ) or slightly developed rhinophores and are easily mistaken for L. capitata or L. depressa.
2) Extended pale metapodium (tail) is about 13-18.5% of extended body length ( 04 Limapontia senestra. ).
3) Often a small pale dorsal spot and lateral spots form a quincunx or similar; missing on translucent specimens with visible pale viscera ( 24 Limapontia senestra ).
4) Eye patches and rhinophores whitish.
5) Anus a short distance behind midpoint of body (difficult to see when not defecating).
6) Full salinity, lagoons perhaps with salinity c. 20‰, and rock pools up to MHW on exposed coasts.
Similar species
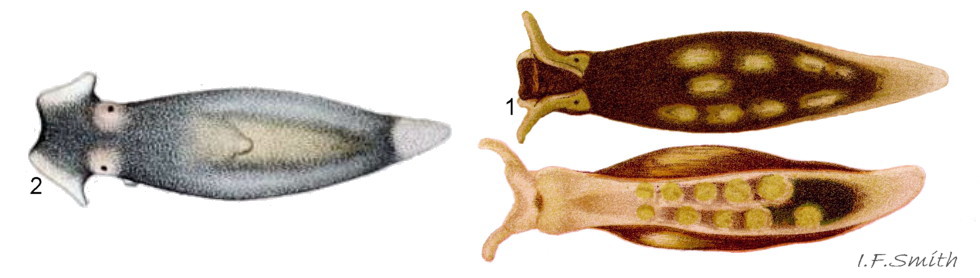
Limapontia depressa Alder & Hancock, 1862 ( 17 Limapontia senestra )
1) No digitiform rhinophores but many have raised ribs/sharp edges to recessed eye-area.
2) Pale metapodium (tail) absent or negligible.
3) Often very small, random, light freckles but no distinct, pale, central mark on dorsum.
4) Pale eye patches.
5) Dorsal anus close to posterior.
6) On saltings with very low salinity, 3‰, to fully marine salinity (though slow to adapt to change in salinity). Sublittoral in inner Baltic.
Limapontia capitata (O. F. Müller, 1774) ( 18 Limapontia senestra )
1) Rhinophoral crest above each eye, no digitiform rhinophore.
2) Substantial pale metapodium (tail) c. 19-25% of extended body length.
3) Usually a large pale mark on the dorsum; visible pale viscera in translucent L. senestra ( 24 Limapontia senestra ) and faded patch on some L. depressa can be mistaken for the dorsal mark of L. capitata.
4) Eye areas and head-crests whitish.
5) Anus a short distance behind midpoint of body.
6) Sublittoral and all levels of the shore in pools and moist positions. Usually on Cladophora attached to hard substrate. Optimum salinity 30‰, can survive 5 to over 40‰, but sustainable population improbable below 10‰, the lower limit for spawning.
Ecology and behaviour
In Britain, L. senestra is found on its food algae, Cladophora spp. ( 19 Limapontia senestra. ), and sometimes on other nearby filamentous species ( 20 Limapontia senestra ), in shaded positions and in rock pools. In the Barents and White Seas, it is frequently on Acrosiphonia (Roginskaya, 200). It lives in the shallow sublittoral and on shores with wave exposure varying from sheltered lagoons to pools on exposed rocks ( 21 Limapontia senestra ). It is usually found in full marine salinity often on exposed coasts in pools at MHW. It lives in the Fleet lagoon, Dorset, at possibly c. 20‰ salinity (Kluijver et al.) (16 Limapontia senestra), but most of the Fleet has fully saline conditions (JNCC).
L. senestra consumes Cladophora spp. by holding a filament vertically in a groove at the front of its head ( 12 Limapontia senestra ) while it punctures it and sucks the cytoplasm from, preferentially, the terminal cells leaving them colourless ( 22 Limapontia senestra ). Its single row of radular teeth, adapted to only slitting and cutting, restricts L. senestra to suctorial feeding. The leading tooth is used to puncture algal cell walls and the newer, unused teeth function as a spear shaft. Worn out older teeth are retained in an ascus sac (Thompson, 1976).
Cladophora spp. have large, coenocytic, multinucleate cells ( 19 Limapontia senestra. ) so there are few internal cell walls subdividing the cytoplasm, which is consequently easily extracted by suction. ’Enteromorpha’, currently genus Ulva, is sometimes mentioned as a food alga (Miller, 1962 and Hayward & Ryland, 2009) but, though it is often found on or near filamentous spp. of Ulva, this is unlikely to be eaten as all species in the order Ulvales, having uninucleate cells (Wichard et al. 2015), are not coenocytic, so unsuitable for suctorial feeding ( 20 Limapontia senestra ). Jensen (1975) observed the related L. capitata to hold filamentous Ulva in the feeding position but fail in its efforts to extract any food.
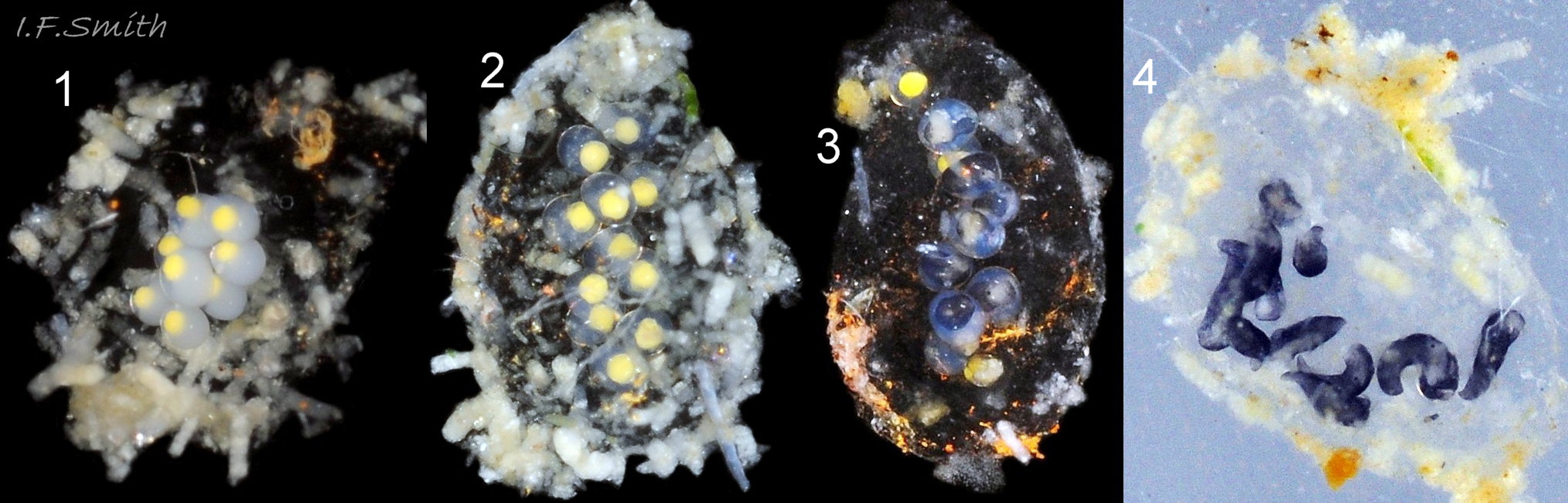
Like other Sacoglossa, L. senestra is a simultaneous hermaphrodite. Its vaginal opening is closed by the bursa copulatrix which forms a swelling on the right side of the body ( 10 Limapontia senestra ). Mutual impregnation is by each partner piercing the other with a sharp hypodermic stylet on the end of the penis ( 09 Limapontia senestra . ) (Gascoigne, 1976). Spawning in Britain is from February to September. Each spawn mass contains up to 40 ova; an unusually small number for British sea slugs, but containing unusually large eggs (diameter 0.4 mm); spawn masses observed in captivity had 7-13 ova per mass (IFS pers. obs.) Initially, ova have a bright yellow yolk surrounded by white albumen, the white part becoming colourless as the albumen is consumed ( 23 Limapontia senestra ). The yellow disappears as the embryos develop within the egg mass, and tiny black slugs with black eyes in white eye patches can be seen before they emerge when c. 0.7 mm long. Unlike the other two species of Limapontia in Britain, there is no planktonic veliger larval stage. L. senestra is the only European sea slug, apart from Runcina coronata which is sometimes found in the same pools ( 21 Limapontia senestra ), to have no trace of a shell at any stage of its embryonic development (Thompson, 1976).
Distribution and status
L. senestra occurs from the White and Barents seas, Arctic Russia, to Bretagne, France. It appears to be absent from the Baltic and continental coast of the southern North Sea; GBIF map www.gbif.org/species/2298917 . It is one of the commonest sea slugs of the littoral zone in the White Sea and the Murman coast, Russia, where it is locally so abundant that sometimes the tufts of algae are turned almost black from the swarms of L. senestra (Roginskaya, 2000). It is widespread around Britain and Ireland, but varies locally and seasonally in abundance. In some places, it is scarcer than L. capitata but in others, such as Orkney, L. senestra is very much the commoner species UK map NBN species.nbnatlas.org/species/NHMSYS0021056303
Acknowledgements
I thank Kathe Jensen and Cynthia D. Trowbridge for their help concerning Sacoglossa species. I am grateful for the use of images by Keith Alexander, Helen Marshall and Malcolm Storey. Any errors or omissions are my (IFS) responsibility.
References and links
Eliot, C.N.E. (1910). A monograph of the British nudibranchiate mollusca. London, Ray Society. Supplementary Volume. archive.org/details/british_nudibranchiate_mollusca_pt8_l…
Gascoigne, T. (1973). A taxonomic note on the genus Acteonia Quatrefages 1844. Proc. Malac. Soc. Lond. 40: 395–398. doi.org/10.1093/oxfordjournals.mollus.a065236
Gascoigne, T. 1975. A field guide to the British Limapontidae and Alderia modesta. J. Conch. Lond. 28: 359 – 364.
Gascoigne, T. 1976. The reproductive systems and classification of the stiligeridae (Opisthobranchia : Sacoglossa). J. Malac. Soc. Aust. 3 (3-4): 157-172.
Hayward, P.J. & Ryland, J.S. (eds.) 1995 and reprints to 2009. Handbook of the marine fauna of North-West Europe. Oxford University Press, Oxford.
Jeffreys, J.G. (1869). British Conchology, vol. 5 London, John Van Voorst. [as Acteonia cocksii]. archive.org/details/britishconcholog05jeffr/page/30/mode/1up
JNCC, Special Areas of Conservation, Chesil and the Fleet, Annex 1: 1150 sac.jncc.gov.uk/site/UK0017076 accessed 11 July 2021.
Kluijver, M.J. de, Ingalsuo S.S. & Bruyne, R.H. de. Mollusca of the North Sea, Limapontia senestra. Marine Species Identification Portal. www.species-identification.org/species.php?species_group=… accessed 11 July 2021.
Miller, M.C. 1962. Annual cycles of some Manx nudibranchs, with a discussion of the problem of the migration. J. Anim. Ecol. 31(3): 545-569 www.jstor.org/stable/2053?seq=1
National Biodiversity Network Atlas,
species.nbnatlas.org/species/NHMSYS0021056303
Quatrefages, A. De. (1844). Sur les gastéropodes phlébentérés. Annls Sci. nat. (Zool.) 1 (3) (First description, as Acteonia senestra, page 142, first illustration fig. iv, plate 3.) www.biodiversitylibrary.org/page/13407269#page/148/mode/1up & www.biodiversitylibrary.org/page/13407269#page/403/mode/1up
Roginskaya, I. 2000. Russian opisthobranchs. Notes on Limapontia senestra (Quatrefages,1844) in White Sea and Barents Sea. (Sacoglossa, Limapontiidae) Nudibranch news 2(12): 56-58. www.seaslugforum.net/pdf/annews2-12.pdf
Smith, I.F. 2014. Rearing and breeding the sacoglossan sea slug, Limapontia senestra (Quatrefages, 1844). Mollusc World 34: 16-18. Conchological Society of Great Britain and Ireland. www.researchgate.net/publication/352982521_Limapontia_sen…
Smith, I.F. 2021. Limapontia capitata (O. F. Müller, 1774) Identification and Biology Limapontia capitata
Thompson, T.E. (1976). Biology of opisthobranch molluscs 1. London, Ray Society.
Current taxonomy; World Register of Marine Species www.marinespecies.org/aphia.php?p=taxdetails&id=140231
Glossary
bursa copulatrix = spermatozoa receiving organ.
coenocytic = (of algae) with parts made up of large, multinucleate masses of cytoplasm enclosed by the wall of each large cell.
cytoplasm = gelatinous liquid that fills the inside of a cell; ‘cell sap’.
digitiform = shaped like a finger.
hermaphrodite, simultaneous = individual acts as both male and female at the same time with similar partner.
metapodium = hind part of the foot.
multinucleate = (of cells) having more than one nucleus per cell, i.e., multiple nuclei share one common cytoplasm.
ovotestis = (pl. ovotestes) hermaphrodite organ serving as both ovary and testis.
propodial = at the front of the foot.
quincunx = pattern of five as on dominoes or dice.
radula = usually a chitinous ribbon with rows of teeth to rasp food, but on Sacoglossa a line of single, fused teeth used like a scalpel to pierce algal cells.
radular = of the radula.
rhinophoral = of a rhinophore or in the usual position of a rhinophore.
rhinophore = chemo-receptor tentacle on the head
spatulate = flattened with a blunt, rounded apex like a spatula.
stylet = hard, sharp, slender piercing structure.
uninucleate = (of cells) having one nucleus per cell.
veliger = mollusc larva, usually with a shell, but not on L. senestra, which moves by action of cilia on a velum (bilobed flap). Stage may be passed in plankton or, as in the case of L. senestra, within liquid-filled egg-capsule.