Click image to enlarge with full caption. Main text below slider.
Differentiating Littorina obtusata sensu stricto (Linnaeus, 1758)
from Littorina fabalis (Turton, 1825).
Ian F. Smith
Abstract
Adult males of Littorina obtusata sensu stricto and L. fabalis (synonym L. mariae) can be reliably differentiated by the forms of the penes on both extracted dead specimens and unsedated intact animals. Differentiation can often be made within a local population on the basis of shell form, size and colour after these features have been correlated with penis forms in a sample of specimens. The shell features can then be used to identify further local specimens, but cannot be relied on at other localities without confirming penis forms there.
Materials, methods, protocols for acquiring experience, environmental associations of phenotypes, and some sample sites are described and illustrated.
Contents
1. Preliminary check.
2. Taxonomic history.
3. Identification resources.
4. Penis examination methods.
5. Differences in penis morphology.
6. Differences in female anatomy.
7. Differences in shell morphology and colour.
7a. Shell colours.
7b. Shell sizes.
7c. Shell spires.
7d. Shell apertures.
8. Phenotypes of different wave exposures.
8a. Sheltered shores.
8b. Semi-exposed shores.
8c. More exposed shores.
9. Collecting and reliable recording.
10. Acknowledgements.
11. References and web links.
12. Glossary.
1. Preliminary check
Lacuna pallidula, detailed account below image flic.kr/p/hTnxJa , has a similar low spire, very similar spawn, and occurs with L. fabalis on Fucus serratus . It has a much thinner shell with widely open umbilicus, contrasting with the thick columella of L. fabalis.
2. Taxonomic history of L. obtusata and L. fabalis.
1758 to 1914: multiple species and varieties named.
1915 to 1965: all species, except Arctic varieties with spires, combined into one species referred to as Littorina obtusata (Linnaeus, 1758) or, mainly by British workers, as L. littoralis auct. For detail of historic nomenclature confusion see caption below image 01 Differentiation L. obtusata/L. fabalis COMPARISON Theodoxus fluviatalis.
1966 to 1988: gradual acceptance of two species, based on penis differences, named L. obtusata sensu stricto (Linnaeus, 1758) and L. fabalis (Turton, 1825), synonym L. mariae Sacchi & Rastelli, 1966. Arctic varieties with pointed spires were included in one or other of the pair on the basis of their penes.
In the following account, any use of “L. obtusata” in the now unaccepted sense of including both species has “sensu lato” or “s.l.” after it. The name with no addition, or sometimes for emphasis with “sensu stricto” or “s.s.”, refers to the now accepted segregated sense.
3. Identification resources
Differentiation of the two species by penis form was first published in 1966 by Sacchi & Rastelli, but did not appear in many identification guides until the late 1980s. Consequently, most pre 1988 works, apart from post 1966 specialist papers, are of limited use. Some later publications, such as Graham (1988), recognise the two species but provide insufficient information and images for discrimination of the many phenotypes. Hayward & Ryland (1990) and Hayward & Ryland (1995 & reprints to 2009), have images www.seawater.no/fauna/mollusca/fabalis.html that confuse www.ispotnature.org/node/646985 as the only L. mariae shell illustrated has features frequent on juveniles of both species, and the L. obtusata image has thick aperture walls more typical of L. fabalis.
Williams (1990) has useful information on forms and ecology. The authoritative volume Systematics and evolution of Littorina by Reid (1996) has fifty A4 pages of detailed description and comparison of all phenotypes and geographical variations of these two species. It is essential reading for those undertaking scientific study of Littorina species www.raysociety.org.uk/publications/zoology/the-systematic… .
4. Penis examination
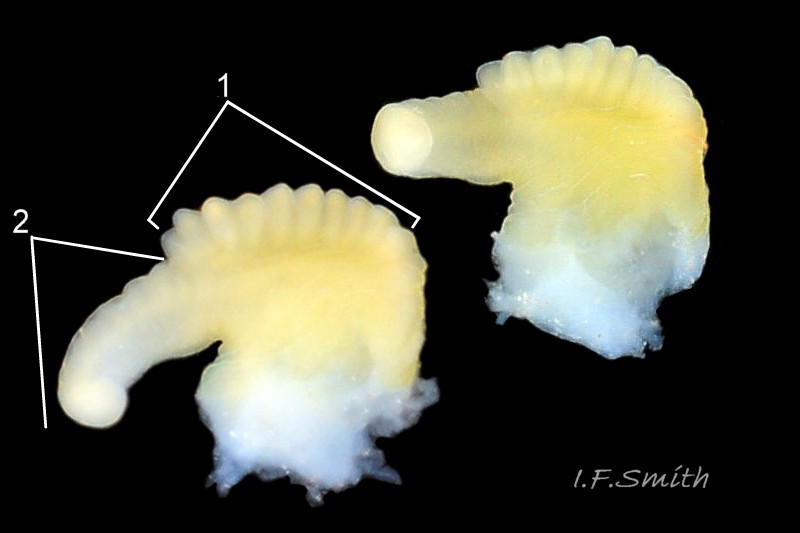
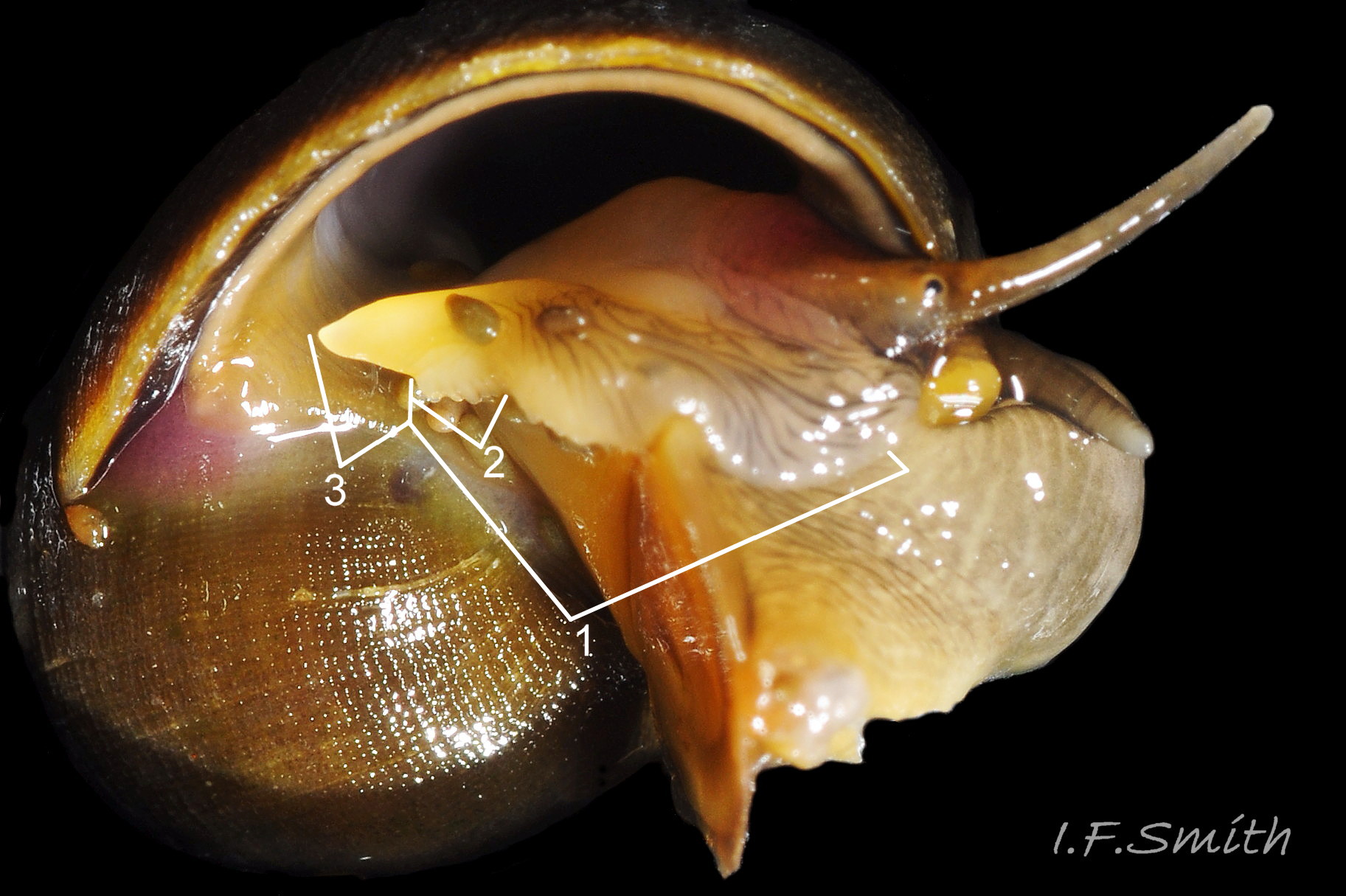
“…only the characters of the adult reproductive system are unequivocal… . …identifications using [shell features] should be confirmed by dissection before routine application in the field” (Reid, 1996). A rare ambiguity in penes linked to hybridization has been reported at one site in Portugal (Carvalho et al., 2016). An obvious penis, positioned at the posterior of the head on the right side
02 Differentiation L. obtusata/L. fabalis , is present on mature males in all seasons, and can be exposed on dead or living specimens.
Dead material
Specimens can be killed instantly by plunging into boiling water, or by leaving in a freezer overnight, or by gradually adding Magnesium chloride or Magnesium sulphate crystals to the water in a small container to first anaesthetize and then kill them. The only dissection required is to carefully crush the shell in a tabletop vice with any rubber protectors removed 03 Differentiation L. obtusata/L. fabalis , avoiding damage to the soft parts, and to pick off the broken fragmets. This method allows unrestricted view of all the necessary details and is quick and reliable, but requires killing of the specimen and destruction of the shell. Alternatively, if boiled for several minutes, the animal can be extracted with a pin from the unbroken shell. Though the penis as a whole is likely to contract on death, especially if boiled, and will be fixed in one of several possible expansions and positions 04 Differentiation L. obtusata/L. fabalis. , its glands are often distended and more easily discerned, and it is likely to resemble published images of dead specimens 04 Differentiation L. obtusata/L. fabalis. .
Living unsedated specimens
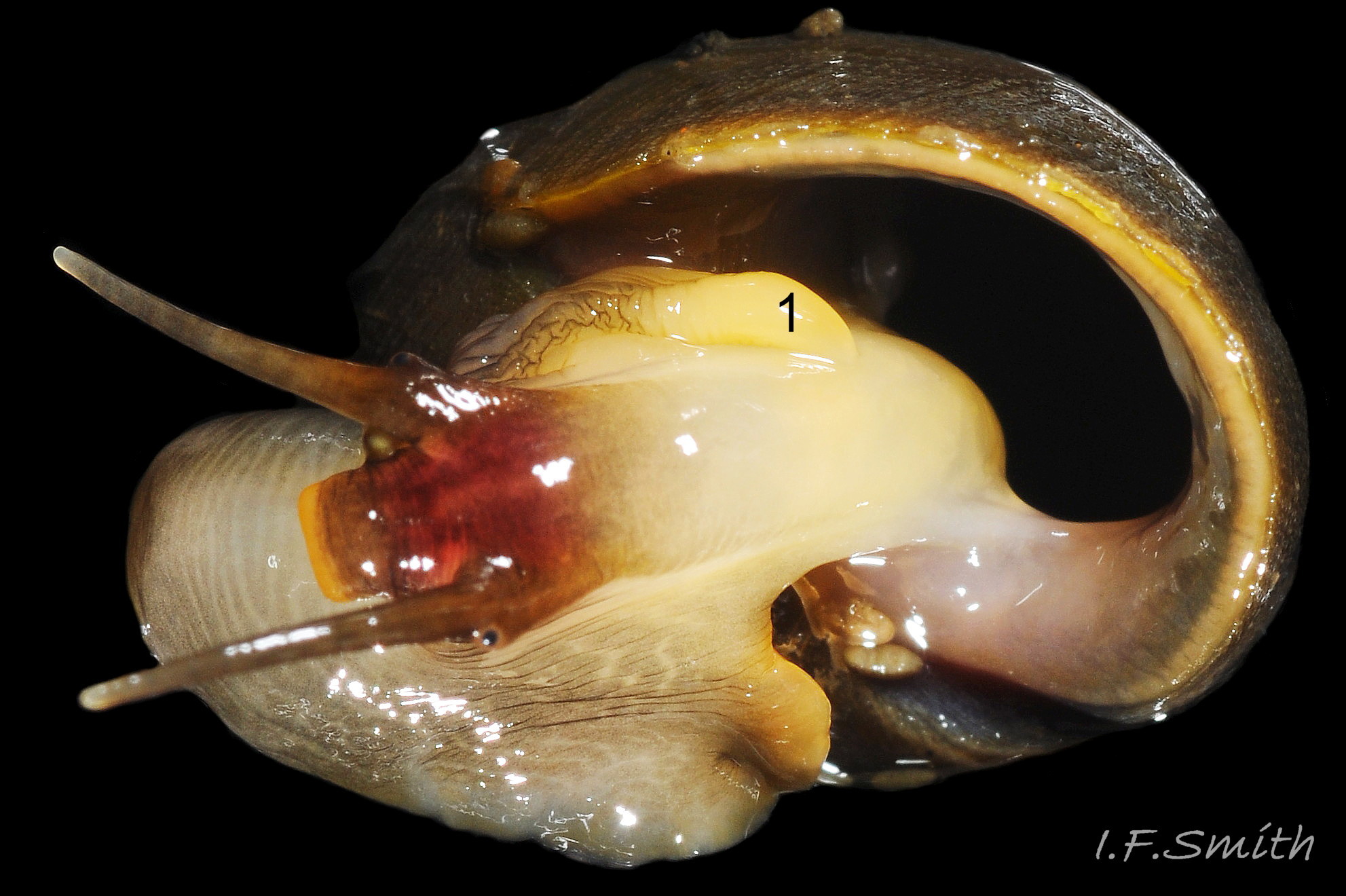
Penes can be examined and photographed on live specimens without harm or anaesthesia using the techniques described in Smith (2012 & 2016 flic.kr/s/aHskNP6GoL ). While live, the penis can be held in a variety of positions 05a Differentiation L. obtusata/L. fabalis & 05b Differentiation L. obtusata/L. fabalis and will often be generally more expanded than on dead material, but the glands are often less prominent than on dissected penes and frequently hidden when held against the body. Time and patience will be required as this pair of species may be reluctant to extend from their shells, and several views or images may be needed to accumulate the necessary detail. Best results are obtained if the examination can be within 48 hours of capture. When not being examined, keep in a refrigerator at about 6°C in tightly closed plastic boxes dampened by, but not awash with, seawater. L. fabalis will extend when immersed and restrained 07 Differentiation L. obtusata/L. fabalis as described in Smith (2012 & 2016), but L. obtusata s.s. exposes itself more readily when damp than when submerged 08 Differentiation L. obtusata/L. fabalis & 09 Differentiation L. obtusata/L. fabalis .
5. Differences in penis morphology,
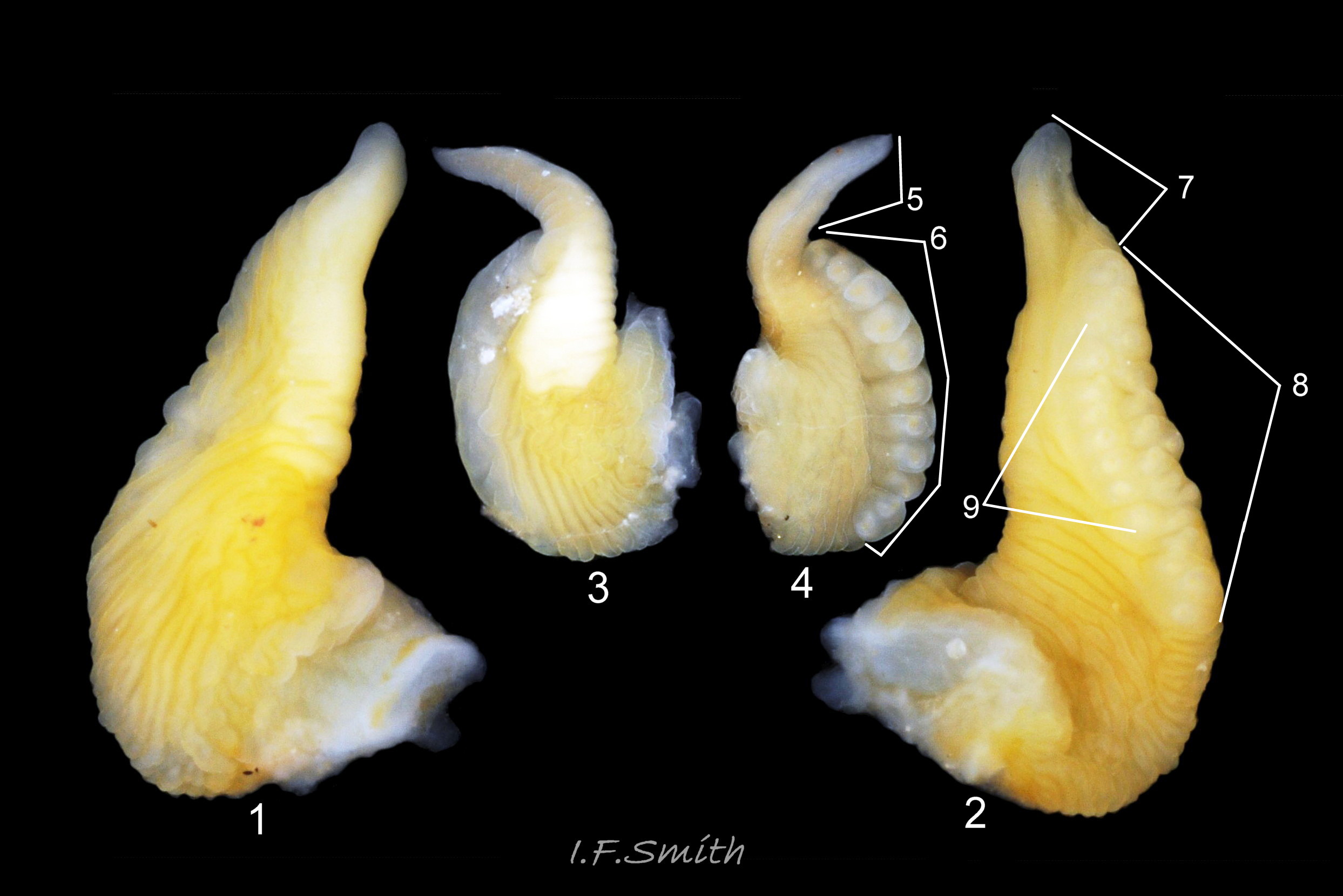
Mamilliform penial glands
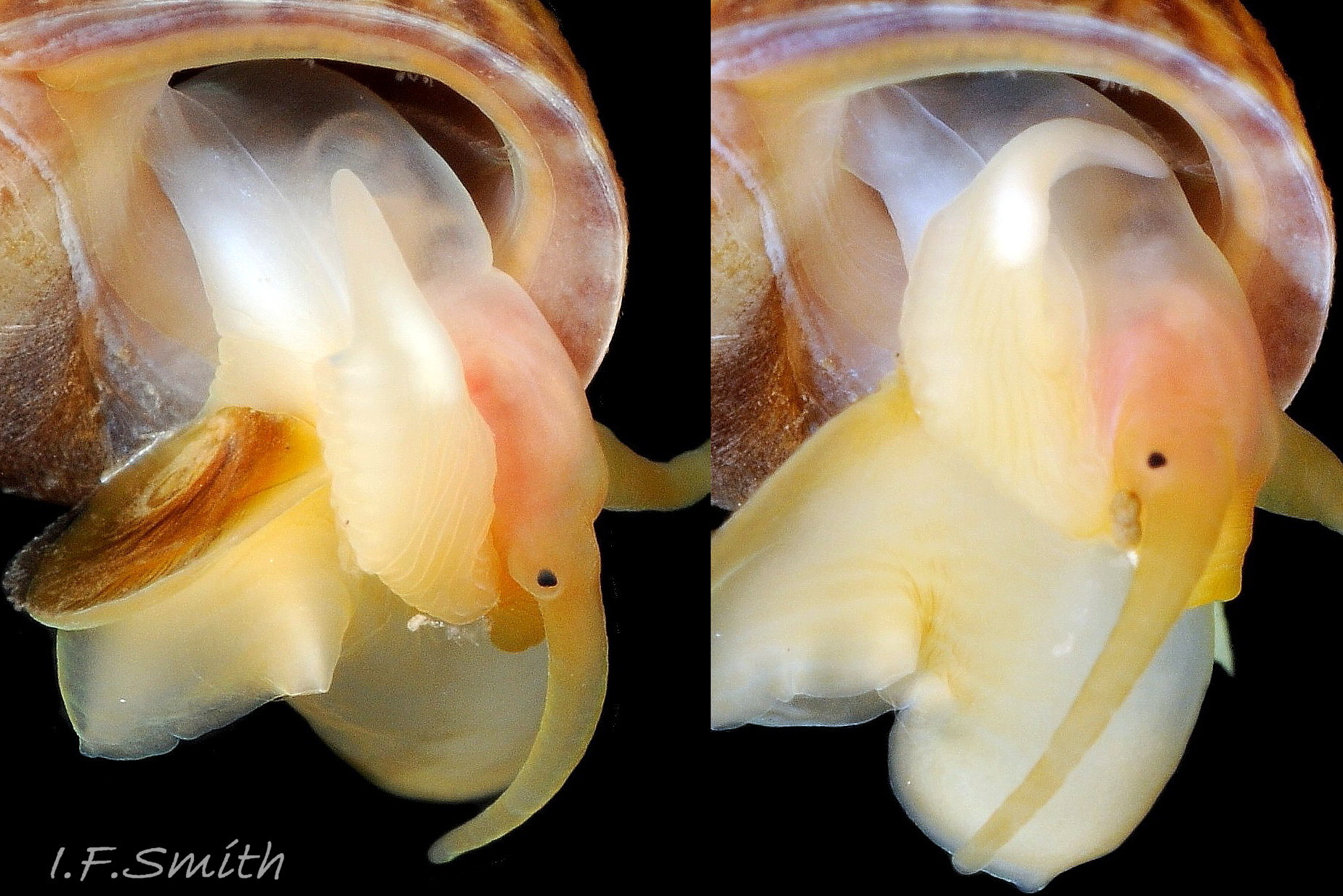
L. fabalis has 3 to 17 large glands in a single row along 40% to 70% of the ventral edge of its penis 10 Differentiation L. obtusata/L. fabalis . The glands are usually visible on live unsedated specimens 07 Differentiation L. obtusata/L. fabalis .
L. obtusata has 10 to 54 closely packed small glands, in one or more irregular rows along 75% to 90% of the length of its penis 10 Differentiation L. obtusata/L. fabalis near its ventral edge and on the mesial face usually held against the body 09 Differentiation L. obtusata/L. fabalis ; sometimes multiple rows occupy 60% of the width of the penis. The small glands often do not protrude into view on live unsedated specimens unless the penis is twisted to expose the proximal face 11 Differentiation L. obtusata/L. fabalis .
Filament (glandless tip of penis).
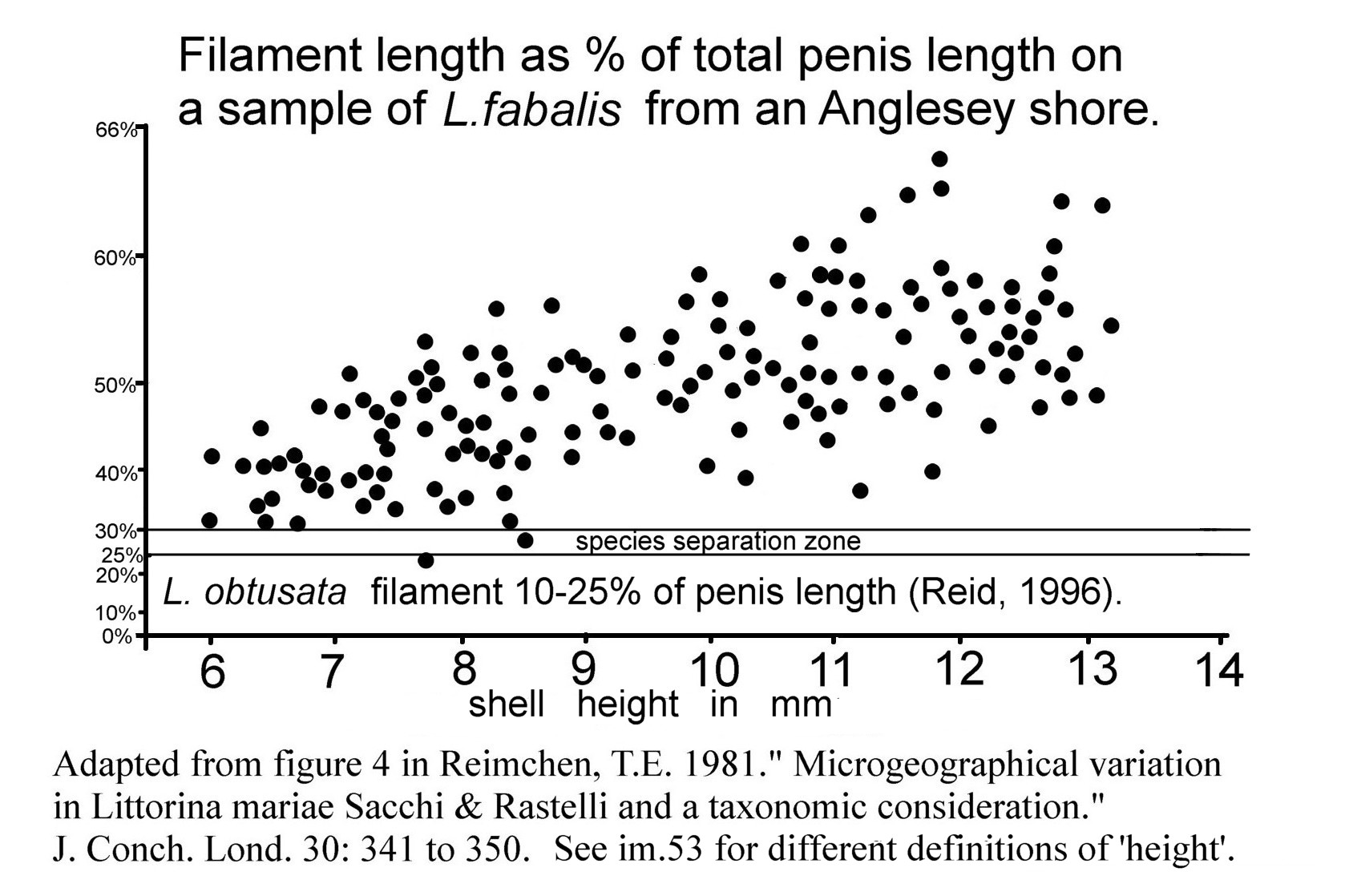
L. fabalis has long and vermiform filament. On dead or anaesthetized specimens filament is 30% to over 60% of total length of penis 10 Differentiation L. obtusata/L. fabalis . Growth is allometric; small individuals frequently have filaments 30% to 40%, while the largest usually have filaments of 50% to >60% of penis length 06 Differentiation L. obtusata/L. fabalis . Penis is motile and varying in length on any individual unsedated specimen 05b Differentiation L. obtusata/L. fabalis , and the appearance is affected by positioning/angle of view, so it is advisable to take several observations or photographs when examining live specimens.
L. obtusata has short triangular filament. On dead or anaesthetized specimens 10% to 25% of total length of penis 10 Differentiation L. obtusata/L. fabalis . On live unsedated specimens it may be difficult to discern the precise limit (distal gland) of the filament because the small glands are hidden. However, being short, wide and triangular, it is obviously different 12 Differentiation L. obtusata/L. fabalis & 13 Differentiation L. obtusata/L. fabalis from the vermiform filament of L. fabalis.
Size of penis depends on the size of the individual male and is not diagnostic of species. On the sheltered Menai Strait, most adult male L. obtusata are larger, and so have larger penes, than most male L. fabalis. On more exposed shores in North Wales, the sizes of the two species can be almost equal.
6. Difference in female anatomy
The copulatory bursa, requires skilled dissection and cannot be examined without killing. It is not illustrated here, see Reid (1996) for details and diagrams.
L. fabalis; half length of jelly gland, not reaching capsule gland.
L. obtusata; extends full length of jelly gland to capsule gland.
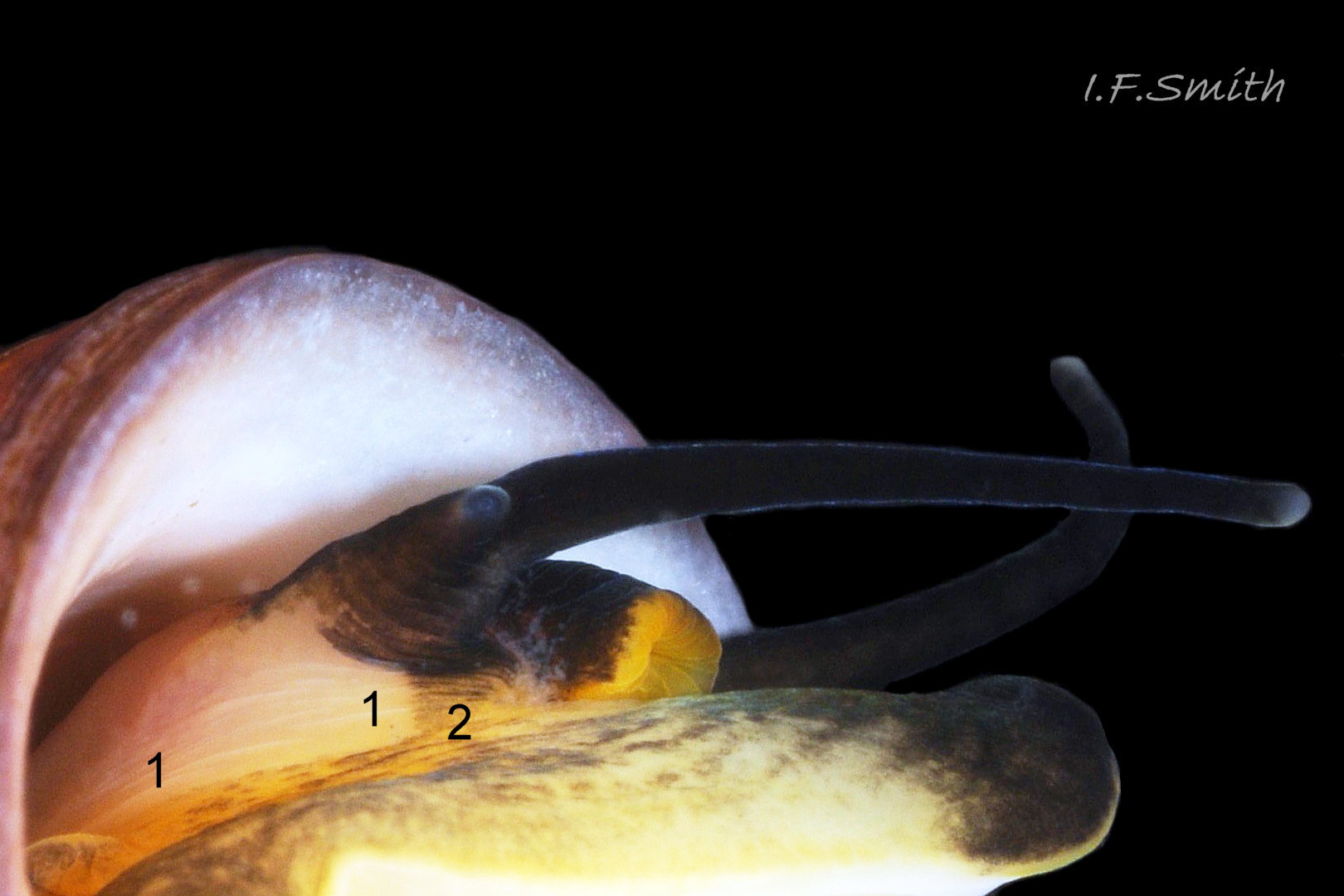
The ovipositor can be observed on live females restrained as described in Smith (2012 & 2016). Goodwin & Fish (1977) stated that, in 99% of cases examined by them in Wales, its colour is “to varying degrees black pigmented” on L. obtusata 14 Differentiation L. obtusata/L. fabalis , while L. fabalis “lacks pigmentation” 15 Differentiation L. obtusata/L. fabalis , but this was not my experience in Wales 16 Differentiation L. obtusata/L. fabalis , and Reid (1996) also found it not always so . The colour is often lost on preserved specimens as the surface frequently sloughs off.
To identify females without dissection, the shell forms and shore zone of each species on the same shore need to be ascertained by examination of some penes on males. This information can also be used to find if local ovipositor colours conform with Goodwin and Fish (1977).
7. Differences in shell morphology and colour
No single shell feature is diagnostic in all situations, and some features intergrade. When several features are considered in combination with habitat detail, it is often possible to give a probable identification, but, for certainty, confirmation by examination of penes is required. Some shores, such as in Denmark and from Kent to Dorset, have problematic shell forms, so penis examination is especially necessary (Reid, 1996). Correlations, validated by penis examination, of species with shell form and colour on specific shores can be found in published previous studies, but should be used with caution as shell form may vary on different parts of the same shore (Reimchen, 1981) and may change from year to year with variation in the climate. Shells of Arctic and subarctic populations differ from those south of the Lofoten Islands; they are less well known and are excluded from this account except where specifically mentioned; further detail in Reid (1996).
7a. Shell colours of both species are often classified with the terms below; percentages are of specimens in a large collection of a mixture of both species from across Britain (Smith, 1976, in Reid, 1996). Some authors give slightly different interpretations of a colour term for each species, and it can be difficult to define the limits of fusca, brownish olivacea and faintly marked dark reticulata. Colours are best viewed on live specimens in water as the shell and the periostracum, which often contributes to the colour, erode and fade readily on dead shells 17 Differentiation L. obtusata/L. fabalis .
Olivacea: 55%. Exterior olive green 18 Differentiation L. obtusata/L. fabalis to olive brown 19Dof 19 Differentiation L. obtusata/L. fabalis , interior often purplish to brownish. Usually the commonest colour form, and almost diagnostic, of L. obtusata on shores well sheltered from waves. An algal coating on L. fabalis 20 Differentiation L. obtusata/L. fabalis may confuse unless scraped off; true olivacea L. fabalis are extremely rare or absent in Britain, but they do exist in at least two places in Galicia, Spain on Zostera.
Reticulata: 33%. Exterior yellow to brown with darker reticulate 21 Differentiation L. obtusata/ L. fabalis , chequered 22 Differentiation L. obtusata/L. fabalis or zig zag 23 Differentiation L. obtusata/L. fabalis. pattern; interior varied, can be white 21 Differentiation L. obtusata/ L. fabalis , sometimes tinted pink or violet and sometimes with exterior pattern showing near rim within aperture 24 Differentiation L. obtusata/L. fabalis . Usually the commonest colour form of both species on shores exposed to wave action.
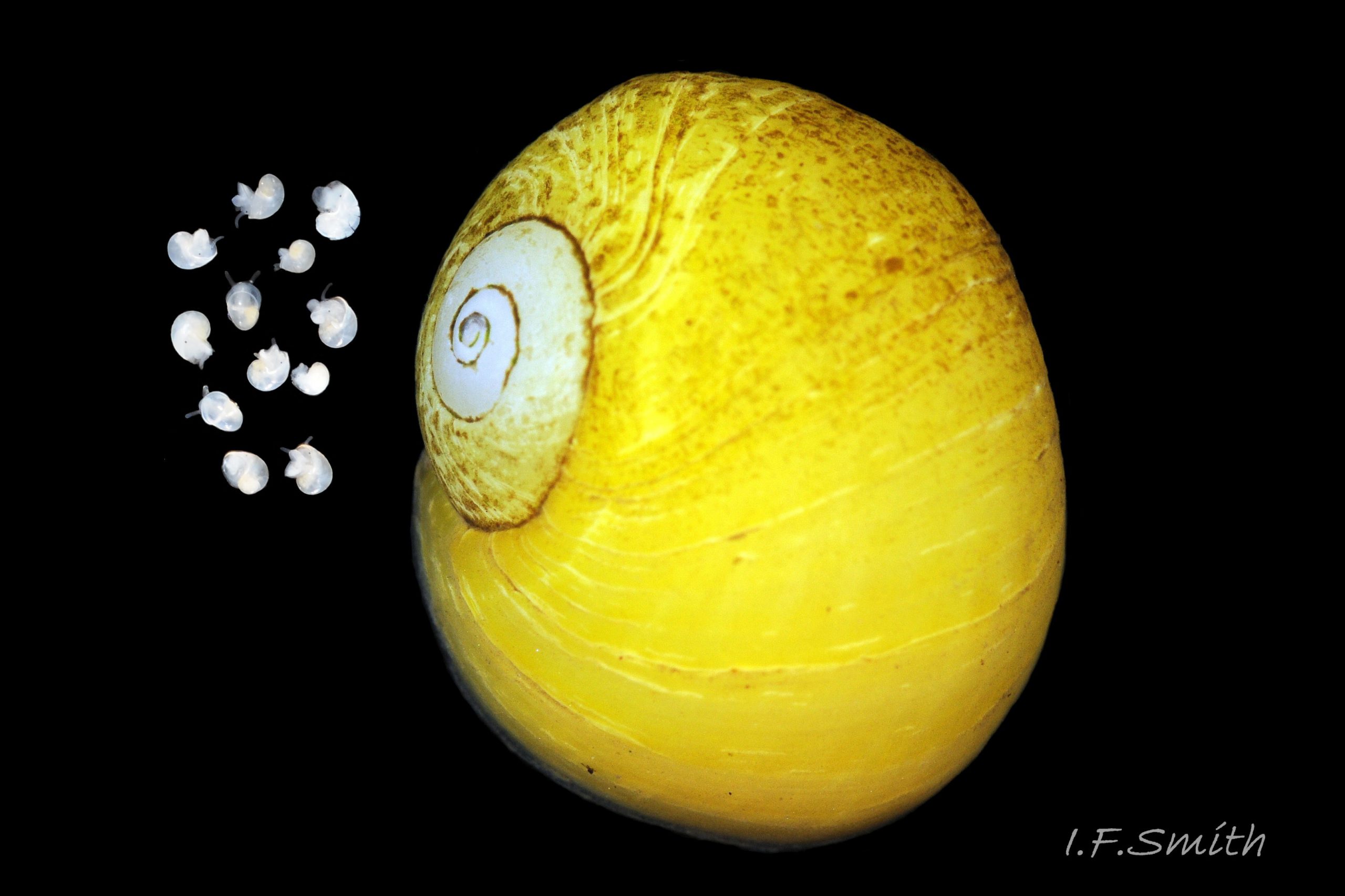
Citrina: 10%. Exterior yellow 25 Differentiation L. obtusata/L. fabalis grading to white; interior white 26 Differentiation L. obtusata/L. fabalis . Usually the commonest colour of L. fabalis on shores well sheltered from waves, but not diagnostic as it also occurs on L. obtusata 27 Differentiation L. obtusata/L. fabalis on the same shores.
Fusca: dark brown to black on L. obtusata 19 Differentiation L. obtusata/L. fabalis ; dark chestnut brown on L. fabalis (Reid, 1996). Rare, generally less than 1%, but 100% of sample of 14 L. obtusata s.l. from a site on brackish Isefjord, Denmark (Rasmussen, 1973). A sample of 8 thin shelled, dwarf, L. fabalis from exposed cliff near Aberdeen all appear to be form fusca, but the specimens, at 28 Differentiation L. obtusata/L. fabalis , were photographed 45 years after death, so the colour may have altered.
Each of following less than 1%.
Aurantia: orange. 29 Differentiation L. obtusata/L. fabalis
Rubens: red or brick red. 30 Differentiation L. obtusata/L. fabalis
Inversicolor: two broad dark bands. 31 Differentiation L. obtusata/L. fabalis
Zonata: single pale band around periphery (“equator”) of bodywhorl. 33 Differentiation L. obtusata/ L. fabalis (right specimen).
Alternata: two pale bands. 33 Differentiation L. obtusata/ L. fabalis (left specimen).
On some shores, juvenile L. fabalis up to 4mm diameter are pure white resembling the 3.5mm diameter spiral tubes of spirorbid worms living on the fronds of Fucus serratus frequented by L. fabalis. With later growth of another colour, the juvenile shell is preserved as a pure white early spire 23 Differentiation L. obtusata/L. fabalis. & 34 Differentiation L. obtusata/L. fabalis . Care is required as the spire of L. obtusata is often eroded to dingy whitish, though careful examination will often show traces of the eroded colour 35 Differentiation L. obtusata/L. fabalis . And populations of L. fabalis lacking white spirorbid like spires can weather to dingy white with traces of colour in the same way as L. obtusata 36 Differentiation L. obtusata/L. fabalis .
7b. Shell sizes
On most shores, L. obtusata has a larger mean height than L. fabalis, but there is usually a large overlap in sizes of the largest L. fabalis specimens and young specimens of L. obtusata 37 Differentiation L. obtusata/ L. fabalis . The mean size of both species varies greatly with local conditions 44 Differentiation L. obtusata/L. fabalis. .
7c. Shell spires
In Britain and most of Atlantic Europe, generally, but not consistently enough for reliable differentiation, L. fabalis has a flat or very low spire 36 Differentiation L. obtusata/L. fabalis and L. obtusata a low 38 Differentiation L. obtusata/L. fabalis or slightly raised domed spire 18Dof 18 Differentiation L. obtusata/L. fabalis , often with an angled shoulder on the body whorl giving a squared or barrel like profile 38 Differentiation L. obtusata/L. fabalis . Juveniles of both species usually have flatter spires than adults 31Dof 31 Differentiation L. obtusata/L. fabalis , 32 Differentiation L. obtusata/L. fabalis , 39 Differentiation L. obtusata/L. fabalis & 50 Differentiation L. obtusata/L. fabalis. .
Occasional specimens 40 Differentiation L. obtusata/L. fabalis or local populations have a protruding pointed spire, especially in sheltered brackish conditions, e.g. L. obtusata var. aestuarii in the tidal River Deben, Suffolk, in Jeffreys (1869) , plate CI at archive.org/stream/britishconcholog05jeffr#page/n489/mode… (now scarce or extinct at that locality). On Arctic and subarctic shores north of the Lofoten Islands, and in Greenland and most of Iceland, many of both species have protruding pointed spires. Only L. obtusata occurs in Atlantic North America; it often has a developed spire in northern Maine and Canada.
7d. Shell apertures
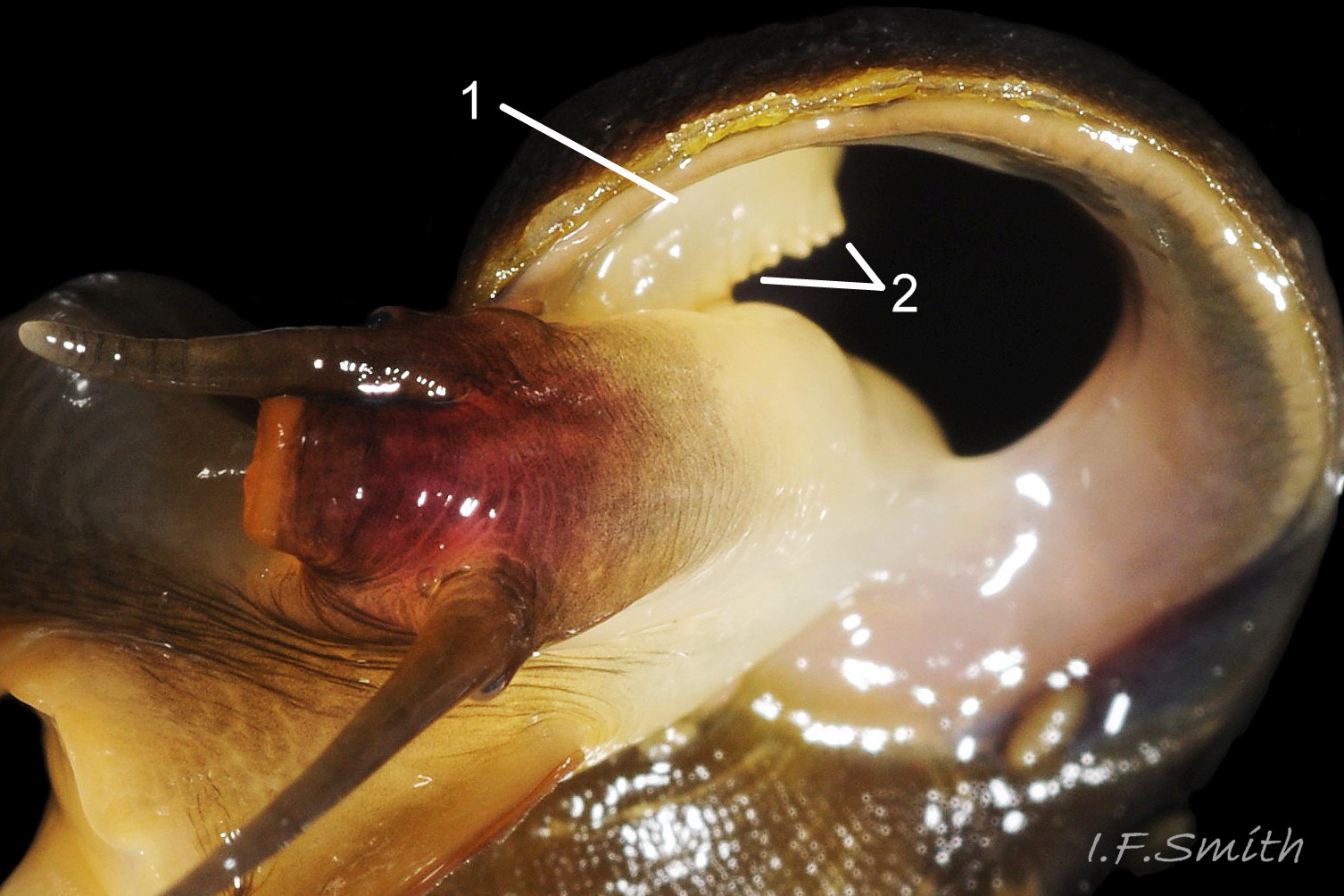
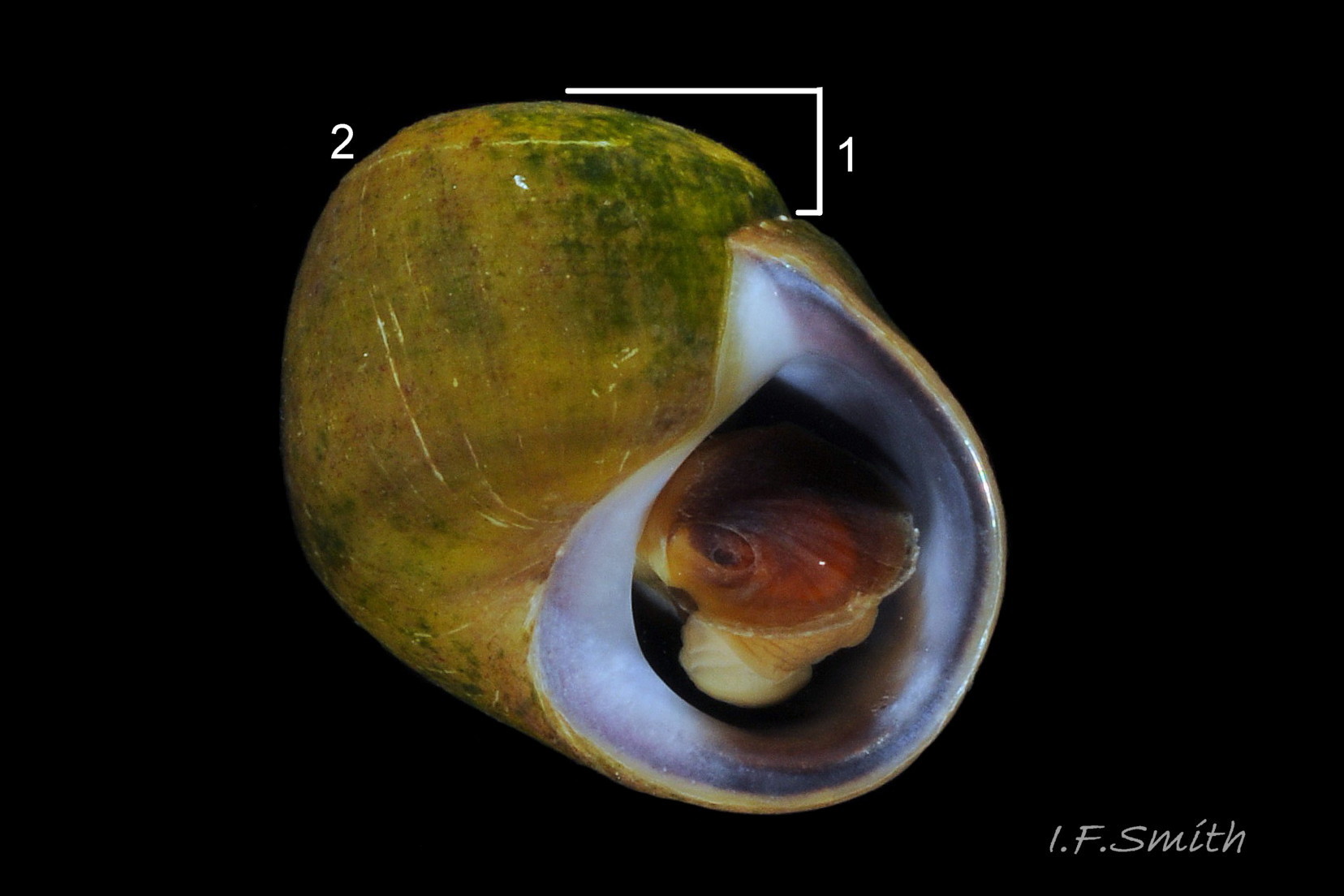
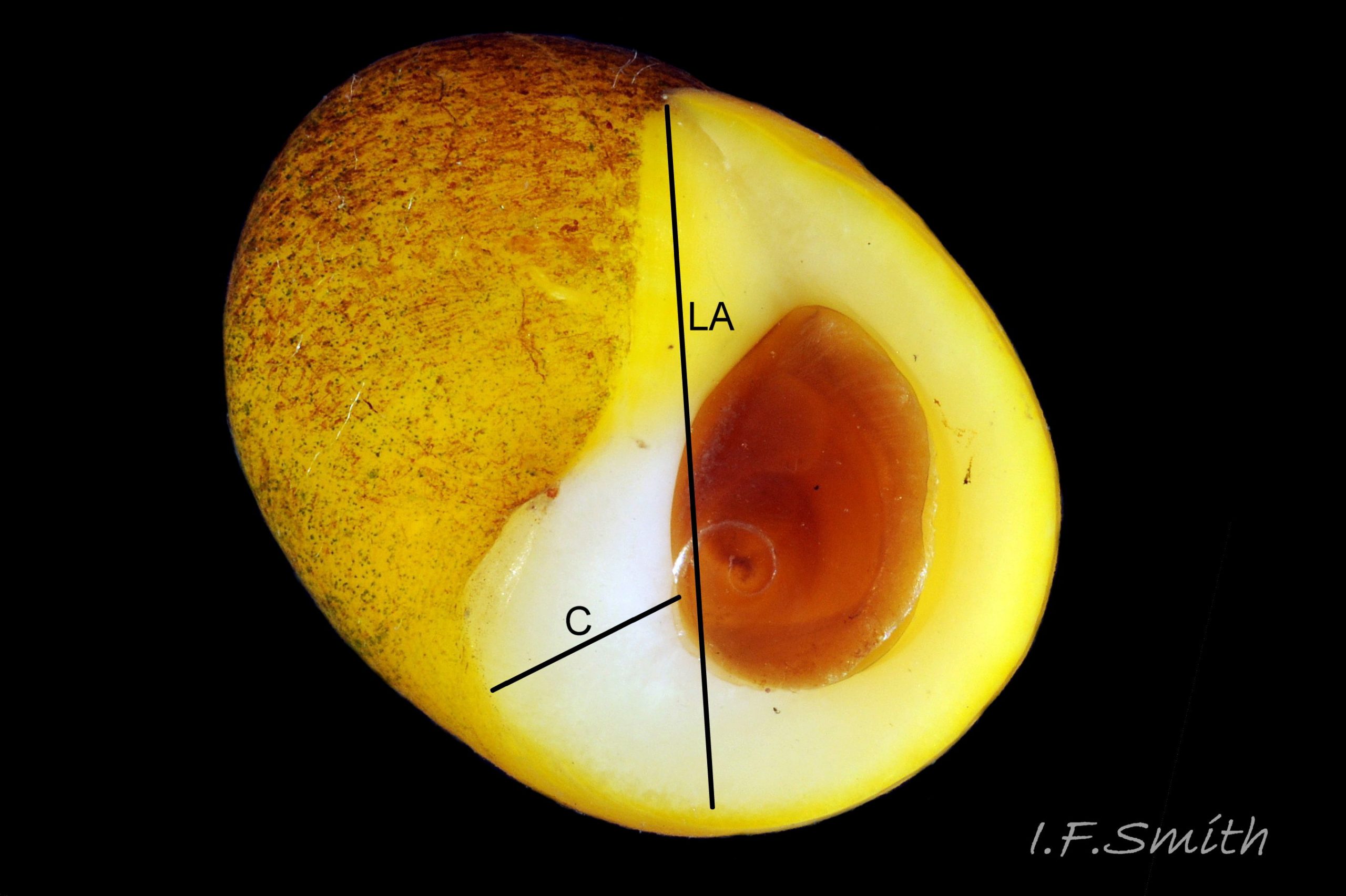
Aperture size is affected by the physical and biological environment, and juveniles have a less expanded body whorl and aperture. The shell walls thicken with maturity in both species, constricting the aperture internally, generally more markedly in L. fabalis, but varying in degree with environment. The most useful measure of shell thickness is that of the lip at the base of the columella (C) divided by the length of the aperture (LA) 41 Differentiation L. obtusata/ L. fabalis . In a sample of 59 adult shells, including less frequent extreme forms, C/LA was usually less than 0.29 on L. obtusata and greater than 0.29 on L. fabalis, but only with a 75% accuracy (Reid, 1996). Accuracy was greater if extreme forms were excluded. Juveniles of the two species are often very similar with thin shell walls, a sharp, fragile aperture rim and an unconstricted opening, and often the anterior (base of aperture) is drawn out into a moderate spout. As small juveniles lack penes, identification cannot always be verified. Association with verified adults, and shell size and colour, if on a shore where there is a large interspecific difference in these features, are usually the best guide 31 Differentiation L. obtusata/L. fabalis & 32 Differentiation L. obtusata/L. fabalis .
Graham (1988) states that “a notch where the outer lip and last whorl meet” is indicative of L. obtusata, but its presence varies with age/spire development and it is not a reliable predictor of species (D. Reid, in litt.) 42 Differentiation L. obtusata/L. fabalis & 43 Differentiation L. obtusata/L. fabalis .
8. Phenotypes of different wave exposures
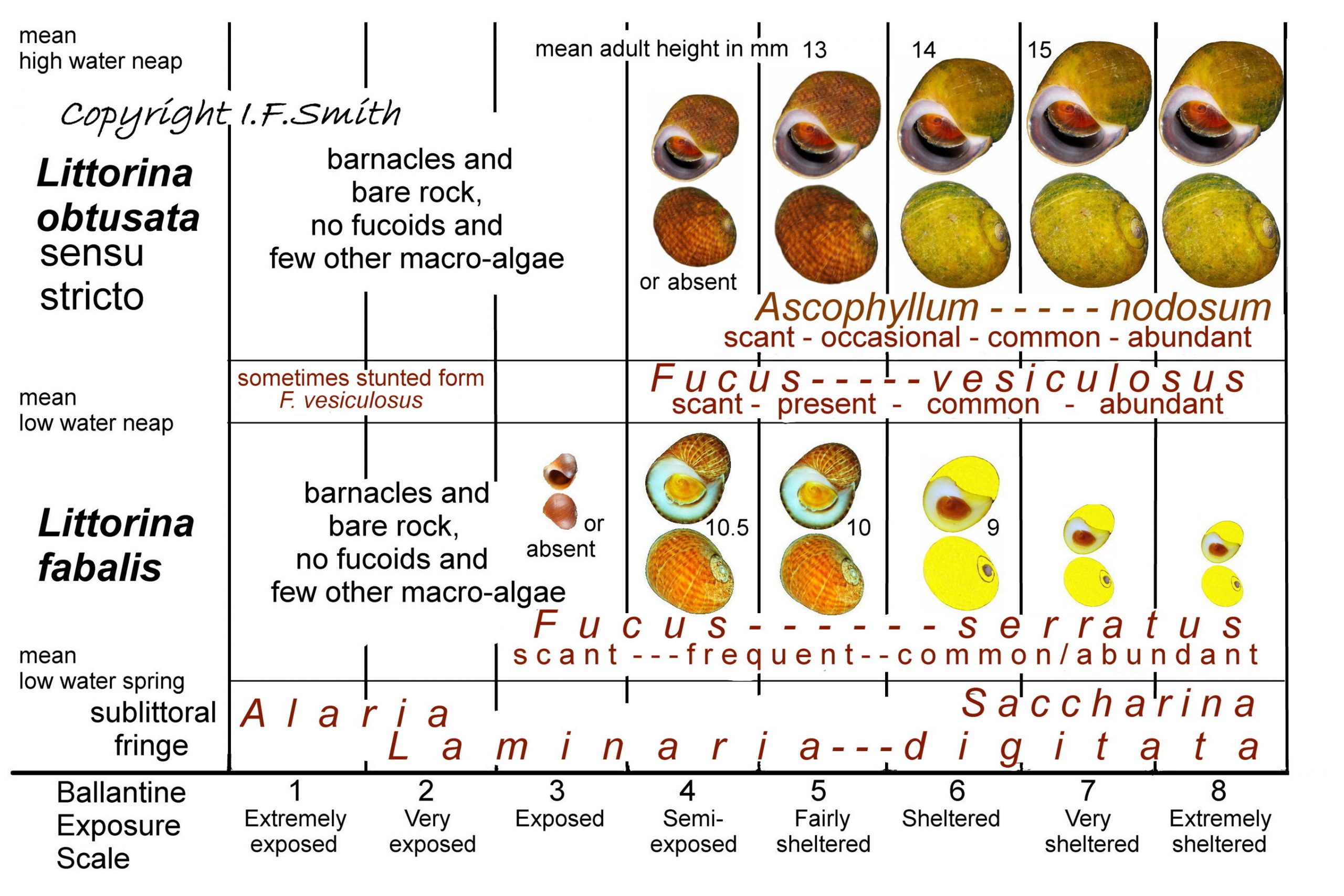
On British rocky shores that have full marine salinity and fucoid algal growth, shell sizes and dominant colours of L. obtusata and L. fabalis vary with the degree of wave exposure. Image 44 Differentiation L. obtusata/L. fabalis. and its caption summarise approximately the most frequent correlations.
8 a. Sheltered shores, not estuarine, scale 8, 7 or 6, are the best for initial experience of differentiating the two species, providing the tidal range is sufficient to clearly separate and define a zone of Ascophyllum nodosum on the middle/upper shore and a zone of Fucus serratus on the lower shore (low spring tide required to expose) 45 Differentiation L. obtusata/L. fabalis. . Olivacea L. obtusata at its largest will probably be on the Ascophyllum, and easily differentiated from citrina L. fabalis at its smallest with thick aperture walls on the Fucus serratus 46 Differentiation L. obtusata/L. fabalis . Unbiased samples can be obtained by shaking plants into a bucket. Juveniles of both species may be yellow and have a similar shape with an anterior spout 37 Differentiation L. obtusata/ L. fabalis , but juveniles from Ascophyllum with adult L. obtusata will likely be that species and be as large as adult L. fabalis, and far larger than tiny juveniles of L. fabalis. If findings are as described, the identifications will almost certainly be correct, but examining the penes will add to experience. Sites with phenotypes as described can be found on the narrow inner portion of the Menai Straits between the two bridges and probably extending to Bangor and Y Felinheli; aerial photograph at data.nbn.org.uk/imt/?mode=SPECIES&species=NHMSYS00210… .
L. fabalis does not eat Fucus serratus, but uses the flat fronds as suitable feeding platforms for its diet of micro epiphytes and detritus. It is sometimes absent in sheltered situations, despite the presence of F. serratus, if excess sediment coats the plants, e.g. possible cause of absence in upper reaches of Severn Estuary (Williams, 1994). Where turbidity and sediment in estuaries 47 Differentiation L. obtusata/L. fabalis or impact of sand laden currents 48 Differentiation L. obtusata/L. fabalis prevent the growth of intertidal fucoid algae, both mollusc species are usually absent or very scarce.
8 b. Semi exposed shores, Ballantine scale 4, will provide experience at the opposite end of the phenotype sequence. Ascophyllum, a favoured food of L. obtusata, will be absent or present as a few scattered stunted plants, and L. obtusata will likewise be absent or present as small specimens on scant Fucus vesiculosus or other algae. Fucus serratus is still usually common at scale 4 and L. fabalis usually achieves its largest size here, sometimes equalling or exceeding any L. obtusata present. Differentiating the species can be very difficult on such shores because their sizes are often similar, the predominant colour form of both species is reticulata, and both tend towards larger apertures with thinner shells. Examination of penes is very necessary. An aerial photograph of this sort of shore open to a fetch across the Irish Sea of 100km to 175km, with a wide wave cut platform and plenty of F. serratus but no observed Ascophyllum, is at data.nbn.org.uk/imt/?mode=SPECIES&species=NHMSYS00210…! 0951H+G!0851H+G Large, reticulate L. fabalis were common here (penes checked). The aperture lip of adults was strongly thickened and the opening correspondingly narrowed. The interior was white 21 Differentiation L. obtusata/ L. fabalis , sometimes tinted pink or violet and the exterior pattern often showed within the rim 49 Differentiation L. obtusata/L. fabalis . No L. obtusata were found because they were absent or not detected among very similar L. fabalis. A thin shelled, wide apertured, citrina juvenile L. fabalis was initially mistaken for L. obtusata, but the white spirorbid like initial whorls and its presence among adult L. fabalis strongly suggested it was L. fabalis 50 Differentiation L. obtusata/L. fabalis. .
8 c. Exposed shores, Ballantine scale 3, 2 & 1, usually lack either species, but L. fabalis may occur in dwarf form on scale 3 shores 51 Differentiation L. obtusata/L. fabalis & 28 Differentiation L. obtusata/L. fabalis if sufficiently moist micro habitats exist with secure refuges for dwarfs to retreat into from violent waves. On some Scottish exposed shores with frequent splash and spray, it “may be found further up on the shore on other fucoids where there is adequate protection from desiccation” (McKay & Smith, 1979). It even sometimes occurs at mean high water neap level on Fucus spiralis (Sacchi, 1969 in Reid, 1996). It also occurs in exposed positions on Mastocarpus stellatus in Galicia, Spain; Palmeria palmata at Grindvik, Iceland; Devaleraea stellatus on Achill Island, Ireland; and on red encrusting algae and bare rock in northern Norway (Reid, 1996). On exposed shores, usually, the aperture is larger and the shell thinner than in other situations, and may resemble L. obtusata s.s. from moderately exposed shores.
In a sample of eight from a scale 3 site near Aberdeen, aerial image at data.nbn.org.uk/imt/?mode=SPECIES&species=NBNSYS00001… , all were fusca dwarfs, height mode 4.2mm, max. 5.5mm, with large apertures and thin shells apart from the wide columellar lip 51 Differentiation L. obtusata/L. fabalis . The spires varied greatly, some, like many L. fabalis, almost flat , others with a large bulging penultimate whorl well proud of the aperture that resembles many L. obtusata s.s..
9. Collecting and reliable recording, suggestions.
# Disregard stranded shells; their anatomy cannot be verified, they lack helpful habitat detail, the periostracum containing much colour may be eroded, and the shell may be bleached and weathered 17 Differentiation L. obtusata/L. fabalis .
# If possible, start with a sheltered shore and then a semi exposed shore to gain experience.
# An individual specimen may have atypical features 51 Differentiation L. obtusata/L. fabalis or be deformed 52 Differentiation L. obtusata/L. fabalis , so examine several; for inspection of penes, eight mature ones give a 99% chance of both sexes if there is a 50:50 ratio.
# To obtain all colours and sizes, collect the sample randomly, e.g. by shaking plants into a bucket. If only the largest are selected by eye, the sample may be biased to females as they are larger than males on average, especially L. fabalis on sheltered shores.
# Take separate clearly labelled samples from discrete habitats e.g. Ascophyllum and F. serratus zones. Be aware that some shores have more than one wave exposure; see Reimchen (1981), link in references.
# Transport specimens in sealed plastic boxes dampened, but not awash, with seawater.
Store in a fridge at about 6ºC and examine as soon as possible; L. obtusata s.s. exposes itself more readily when damp rather than when submerged.
# Check any shell based identification with examination of some penes.
# When submitting records to a recording scheme, make sure to include that the penes were examined so that at a future date your record can be separated from the mass of less reliable records. Check with the scheme organizer that the detail will be included
when entered. Records submitted to the Marine Recorder of the Conchological Society of G.B. & Ireland have anatomical notes included and uploaded as integral parts of the record; contact marine@conchsoc.org . Other detail such as shore exposure and algal zone are also helpful and welcomed. Clear photographs of shell aperture and penes, or preserved specimens, are valued by the Marine Recorder.
10. Acknowledgements
I am indebted to Dr David Reid for his careful examination of this account and for his highly valued advice, but any errors or omissions are my (IFS) responsibility.
I wish to thank Simon Taylor, Marine Recorder for the Conchological Society of G.B. and Ireland, for helpful discussion and the provision of specimens and photographs, and I am grateful to Dr Jan Light for help with literature and specimens.
11. References and web links
Ballantine, W.J. 1961. A biologically-defined exposure scale for comparative description of rocky shores. Field studies 1(3): 1-19. Free pdf at: fsj.field-studies-council.org/media/344345/vol1.3_17.pdf
Carvalho, J., Sotelo, G., Galindo, J. & Faria, R. 2016. Genetic characterization of flat periwinkles (Littorinidae) from the Iberian Peninsula reveals interspecific hybridization and different degrees of differentiation. Biol. J. Linn. Soc., 118: 503 to 519. onlinelibrary.wiley.com/doi/10.1111/bij.12762/abstract
Goodwin, B.J & Fish, J.D. 1977. Inter- and intraspecific variation in Littorina obtusata and L. mariae (Gastropoda, Prosobranchia). J. Moll. Stud. 43: 241 to 254. Extract at mollus.oxfordjournals.org/content/43/3/241.extract
Graham, A. 1988. Molluscs: prosobranch and pyramidellid gastropods: keys and notes for the identification of the species. Brill & Backhuys, for Linn. Soc. Lond. & Estuarine and Brackish-water Sciences Assoc. Synopses of the British Fauna (New Series) no.2. Edition 2 (662pages). Leiden.
Hayward, P.J. & Ryland, J.S. (eds.) 1990. The marine fauna of the British Isles and North-West Europe. Volume 2. Clarendon Press, Oxford.
Hayward, P.J. & Ryland, J.S. (eds.) 1995 and reprints to 2009. Handbook of the marine fauna of North-West Europe. Oxford University Press, Oxford.
Jeffreys, J.G. 1865. British conchology. vol.3. London, van Voorst. (L. fabalis included in L. obtusata as var. fabalis.)
archive.org/stream/britishconcholog03jeffr#page/356/mode/1up
Jeffreys, J.G. 1869.British conchology. vol.5. London, van Voorst. Plate ci, of Littorina obtusata var. aestuarii (As Littorina aestuarii) from Shottisham Creek, R. Deben, near Felixstowe, Suffolk. Abundant there between tide marks. Well developed spire. (Now extinct or nearly so.) archive.org/stream/britishconcholog05jeffr#page/n489/mode…
McKay, D.W. & Smith, S.M. 1979. Marine Mollusca of East Scotland. Royal Scottish Museum, Edinburgh. [An early adoption of the differentiation as L. littoralis (for L. obtusata s.s.) and L. mariae (for L. fabalis) before nomenclature became settled. Maps reliable as all entries made or vetted by the experienced authors.]
Reid, D.G. 1996. Systematics and evolution of Littorina. Ray Society, London.
www.raysociety.org.uk/publications/zoology/the-systematic…
Reimchen, T.E. 1981. Microgeographical variation in Littorina mariae Sacchi & Rastelli and a taxonomic consideration. J. Conch. Lond. 30: 341 to 350.
www.web.uvic.ca/~reimlab/Microgeographical%20Variation-00…
Sacchi, C. F. & Rastelli, M. 1966. Littorina mariae, nov. sp.: les differences morphologiques et ecologiques entre “nains” et “normaux” chez l’espece L. obtusata (L.) (Gastr., Prosobr.) et leur signification adaptive et evolutive. Atti Soc. Ital. Sci. Nat. 105: 351 to 369.
Smith, D.A.S., 1976. Disruptive selection and morph-ratio clines in the polymorphic snail Littorina obtusata (L.) (Gastropoda: Prosobranchia). J. Mollus. Stud. 42: 114 to 135.
Smith, I.F. 2012. Anatomy of marine gastropods without dissection.
Mollusc World 28: 13 to 15. Conch. Soc. GB & Ireland.
Smith, I.F. 2016. Revision of Smith, I.F. 2012. Anatomy of marine gastropods without dissection. flic.kr/s/aHskNP6GoL and pdf version at www.researchgate.net/publication/310467378_Anatomy_of_mar…
Williams, G.A. 1990. The comparative ecology of the flat periwinkles, Littorina obtusata (L.) and L. mariae Sacchi et Rastelli in Field Studies 7: 469 to 482. fsj.field-studies-council.org/browse-by-category/marine-b… (scroll down to 1990) [Fig. 2 has an exposure scale in reverse order of Ballantine scale so 1 = Ballantine 8; and caption error: closed boxes are L. obtusata, open boxes are L. mariae/fabalis.]
Williams, G.A. 1994. Variation in populations of Littorina obtusata and Littorina mariae (Gastropoda) in the Severn Estuary. Biol J Linnean Soc 51: 189 to-198.
onlinelibrary.wiley.com/doi/10.1111/j.1095-8312.1994.tb00…
12. Glossary
3Dof = Image 3 in Flickr album.
allometric (adj.) = of disproportionate growth of a part or parts of an organism as the whole organism grows.
aperture = mouth of gastropod shell; outlet for head and foot.
coll. = in the collection of (named person or institution; compare with leg.).
columella = solid or hollow axial “little column” around which gastropod shell spirals; hidden inside shell, except on final whorl next to lower part of inner lip of aperture where hollow ones may end in an umbilicus or siphonal canal.
columellar (adj.) = of or near central axis of coiled gastropod.
columellar lip = lower (abapical) part of inner lip of aperture.
columellar muscle = large muscle connecting foot/head of gastropod to its shell at the columella.
distal = positioned or facing away from midline of body.
epiphyte = an organism growing on a larger plant for support, but not nutrition.
epizooic (adj.) = of non-parasitic organisms living on surface of animals.
filament = a slender threadlike object or fibre in animal or plant structures.
filament (in Reid, 1996) = glandless tip of penis a.k.a. distal tubule. Often not threadlike.
flagellum = threadlike organ or appendage.
height (of gastropod shells) = distance from apex of spire to base of aperture along/parallel to axis of coiling (see 38 Differentiation L. obtusata/L. fabalis ).
in litt. (abbreviation of Latin “in litteris”) = in unpublished correspondence.
leg. (abbreviation of legit) = collected/ found by (compare with coll.)
mamilliform = shaped like a nipple.
mesial = facing towards the midline of the body.
mobile = able to move self, e.g. a horse, or to be moved by an agent, e.g. a wave or a cell phone.
motile = moves self spontaneously without volition; applicable to whole animals or to parts of them such as cells, gametes, penes or cilia.
penes (plural of penis) = male copulatory organs.
phenotype = form of a species resulting from influence of particular environmental factors.
proximal = towards the centre of the body or point of attachment.
sensu lato (abbreviation s.l.) = in the wide sense, possibly an aggregate of more than one species.
sensu stricto (abbreviation s.s.) = in the strict sense, excluding species that have been aggregated or confused with it.
spire = all whorls of a gastropod shell, except the final body whorl. But in this account, “spired shells” are taken to be those with a pointed apex protruding beyond the body whorl rather than the domed spire of some that extends beyond the body whorl.
taxonomic (adj.) = of or relating to taxonomy.
taxonomy = the description, identification, naming, and classification of organisms.
vermiform = shaped like a worm.