Click image to enlarge with full caption. Main text below slider.
Patella vulgata Linnaeus, 1758
Synonyms: Patella conica Anton, 1838; Patella hypsilotera Locard 1891; Patella radiata Perry, 1811; Patella servaini Mabille, 1888.
Current Taxonomy WoRMS https://www.marinespecies.org/aphia.php?p=taxdetails&id=140685
Vernacular names: Common limpet, Common European limpet, Atlantic limpet (English); brenigen, Llygad maharen (Welsh); patelle de l’Atlantique, patelle commune, bernique (French); gewöhnliche Napfschnecke, gemeine Napfschnecke (German), albueskæl (Danish), albusnegl, olbogesnigel (Norwegian); lapa (Portuguese and Spanish); stor skålsnäcka (Swedish).
Meaning of name:Patella (Latin) = little pan, vulgata (Latin) = common.
GLOSSARY below.
Shell Description
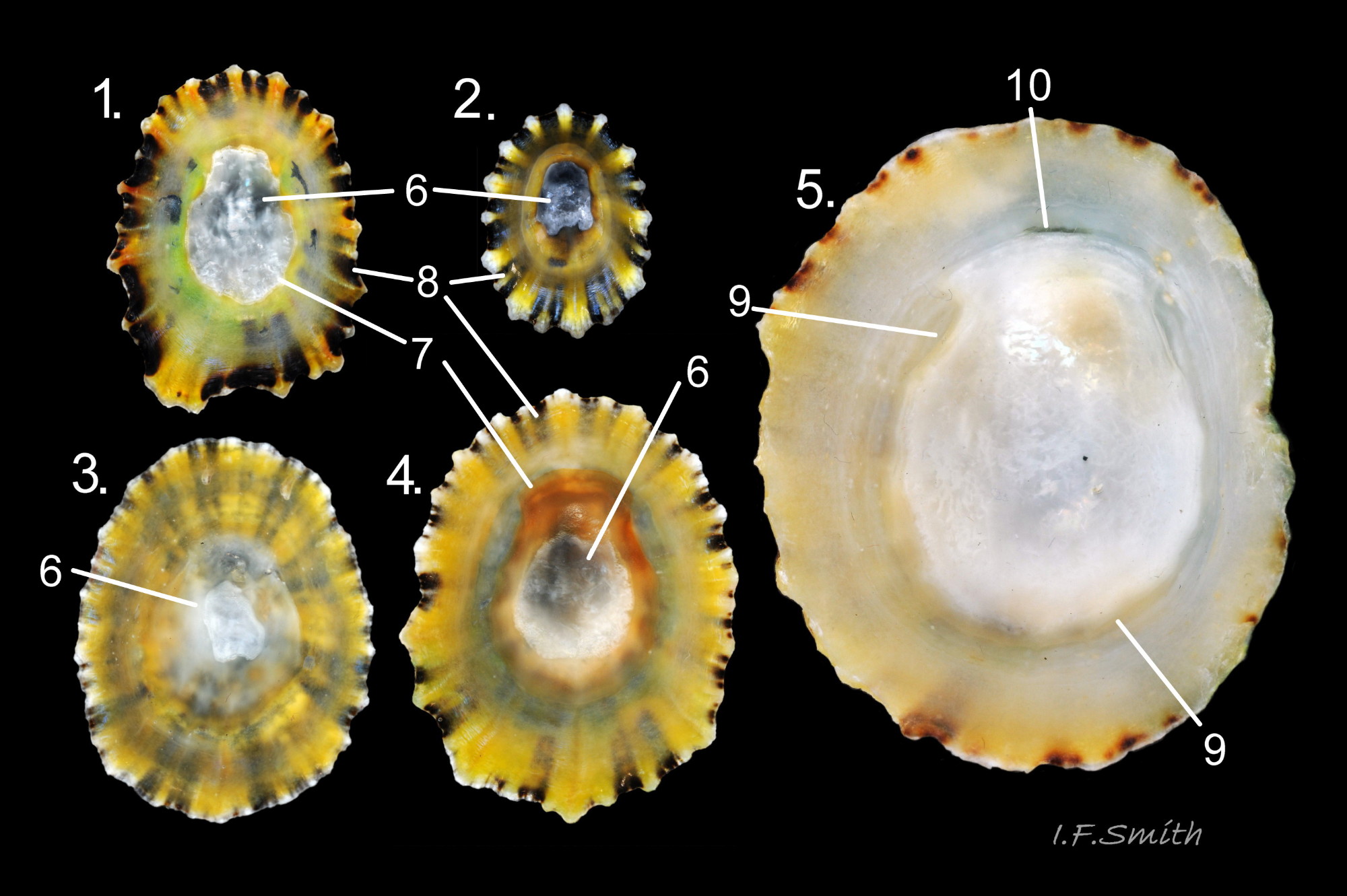
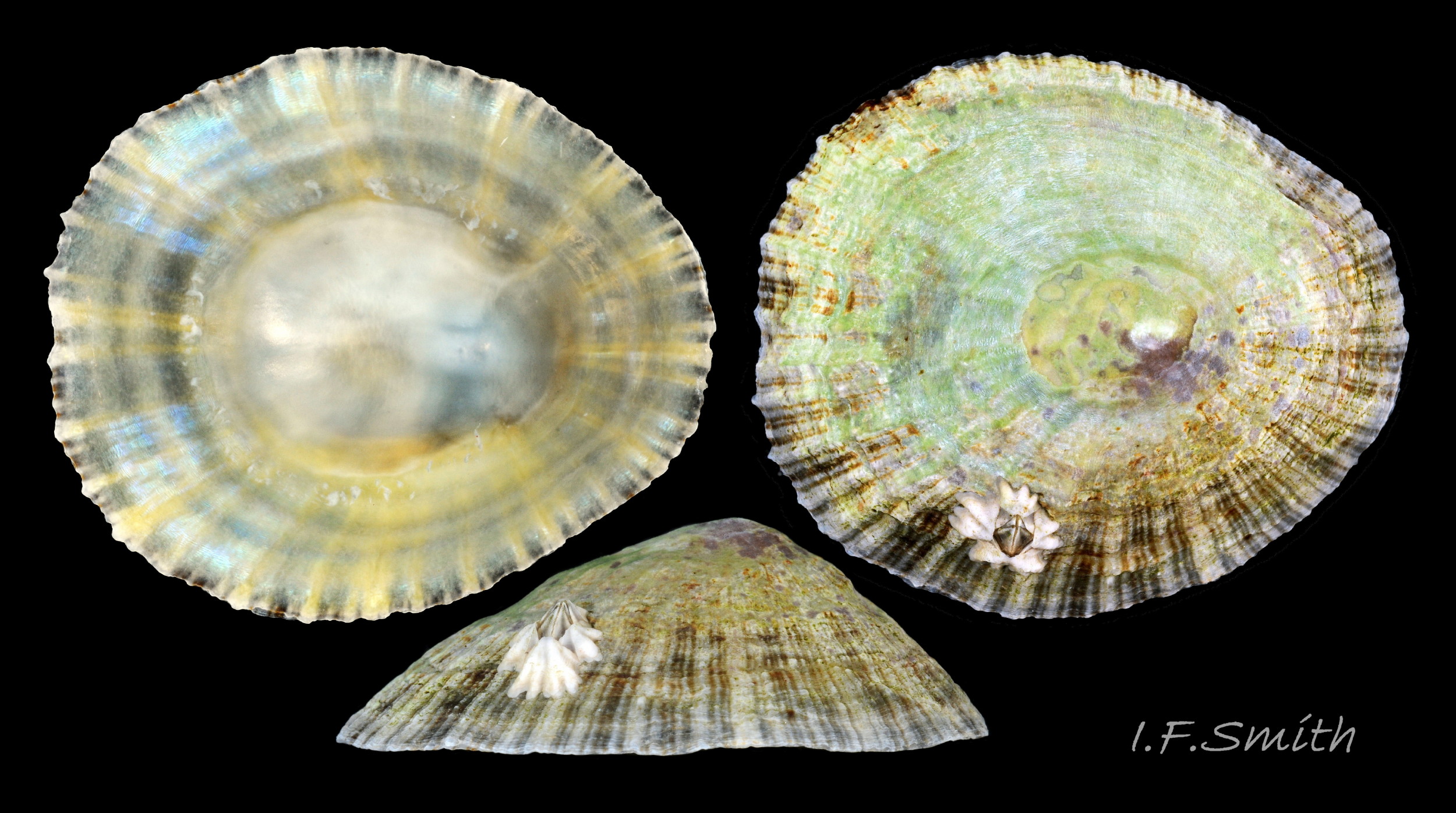
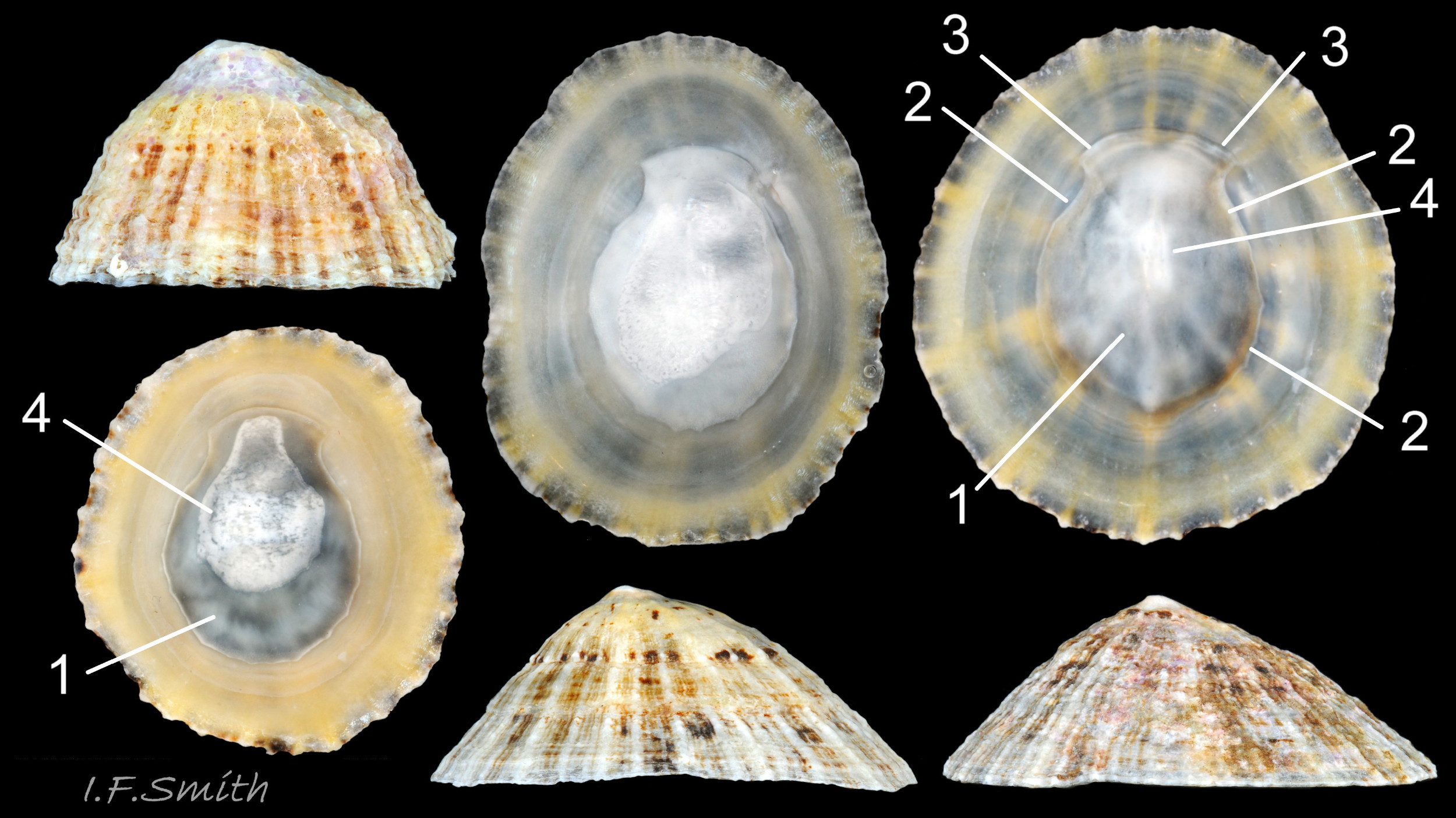
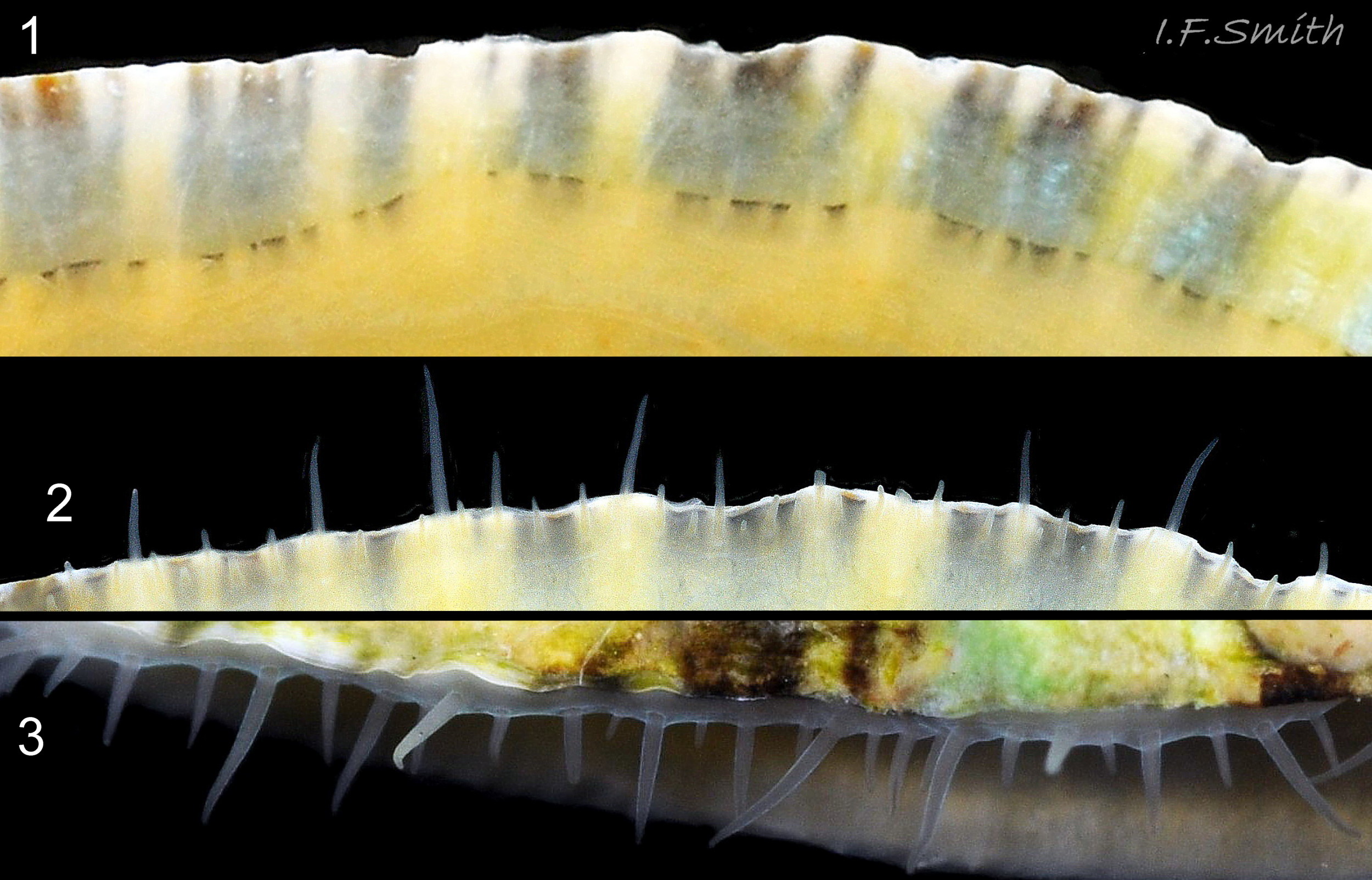
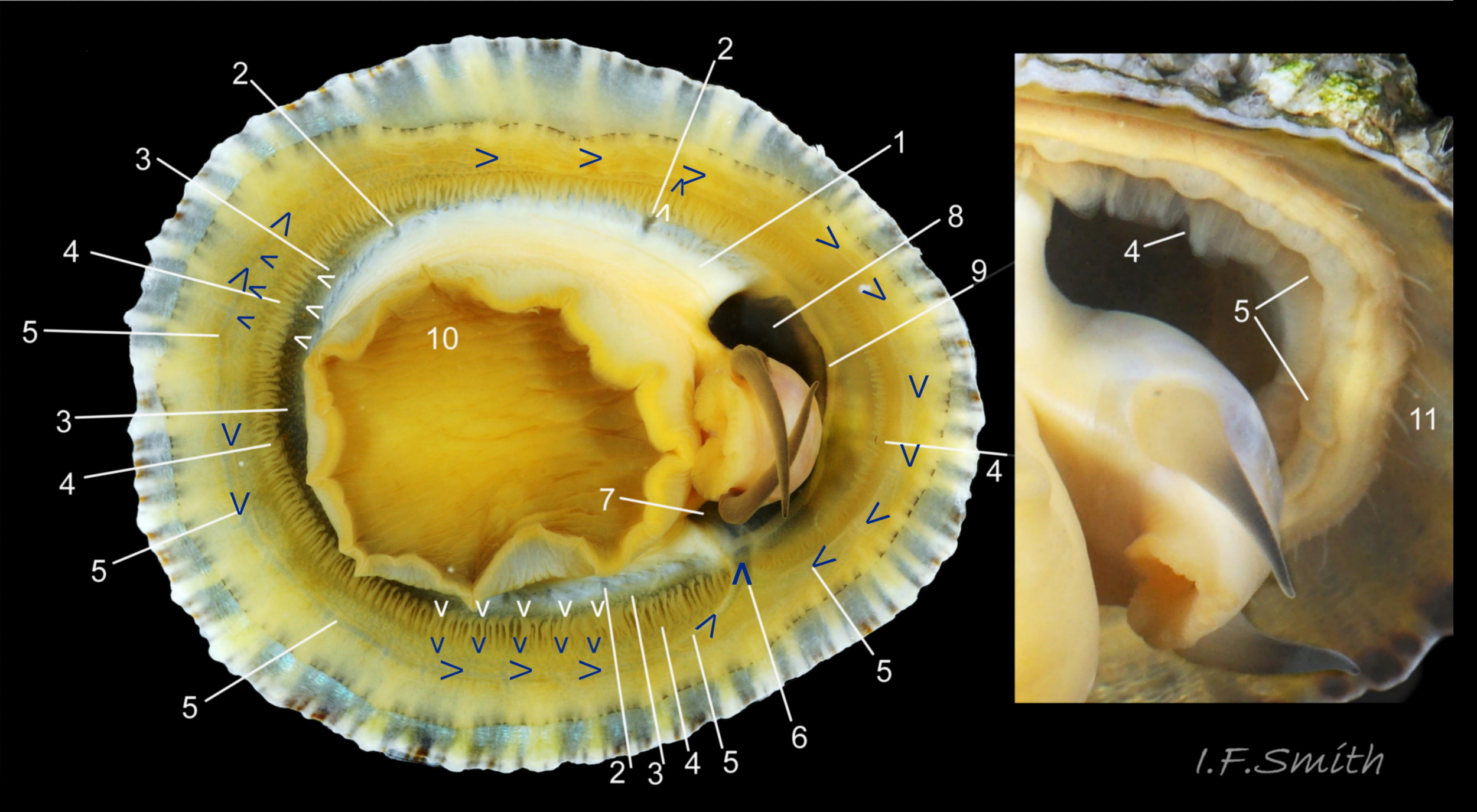
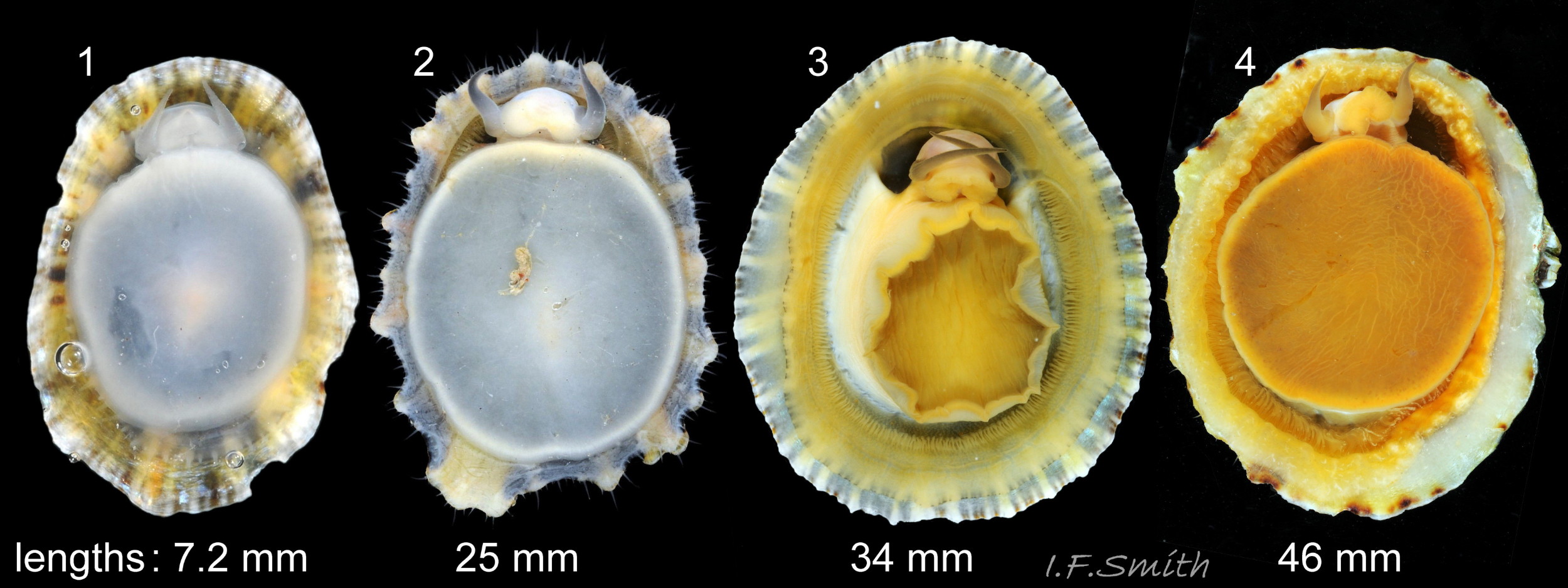
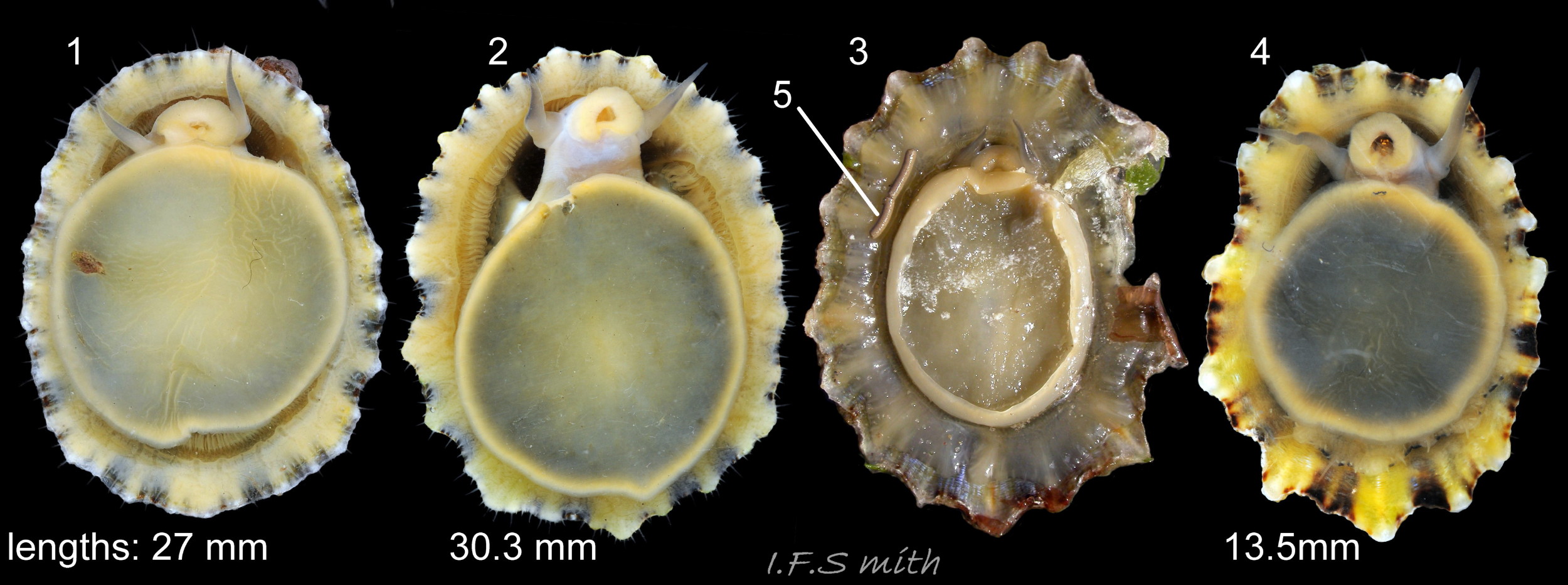
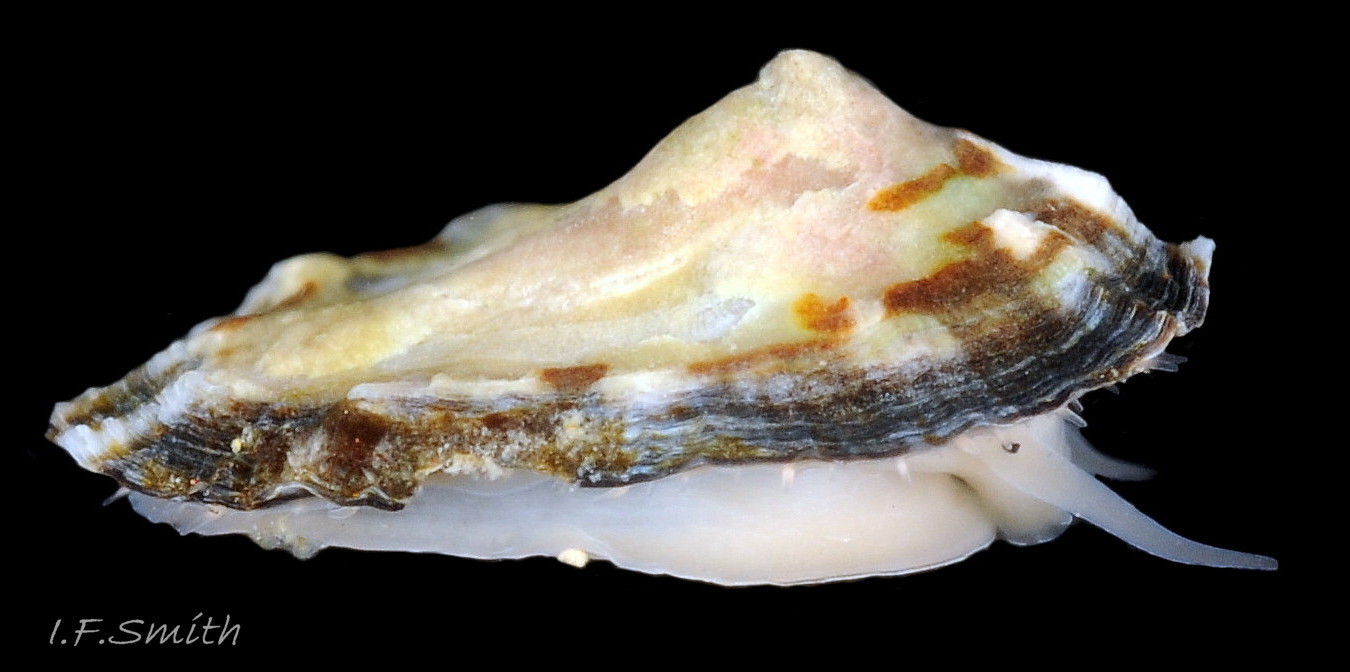
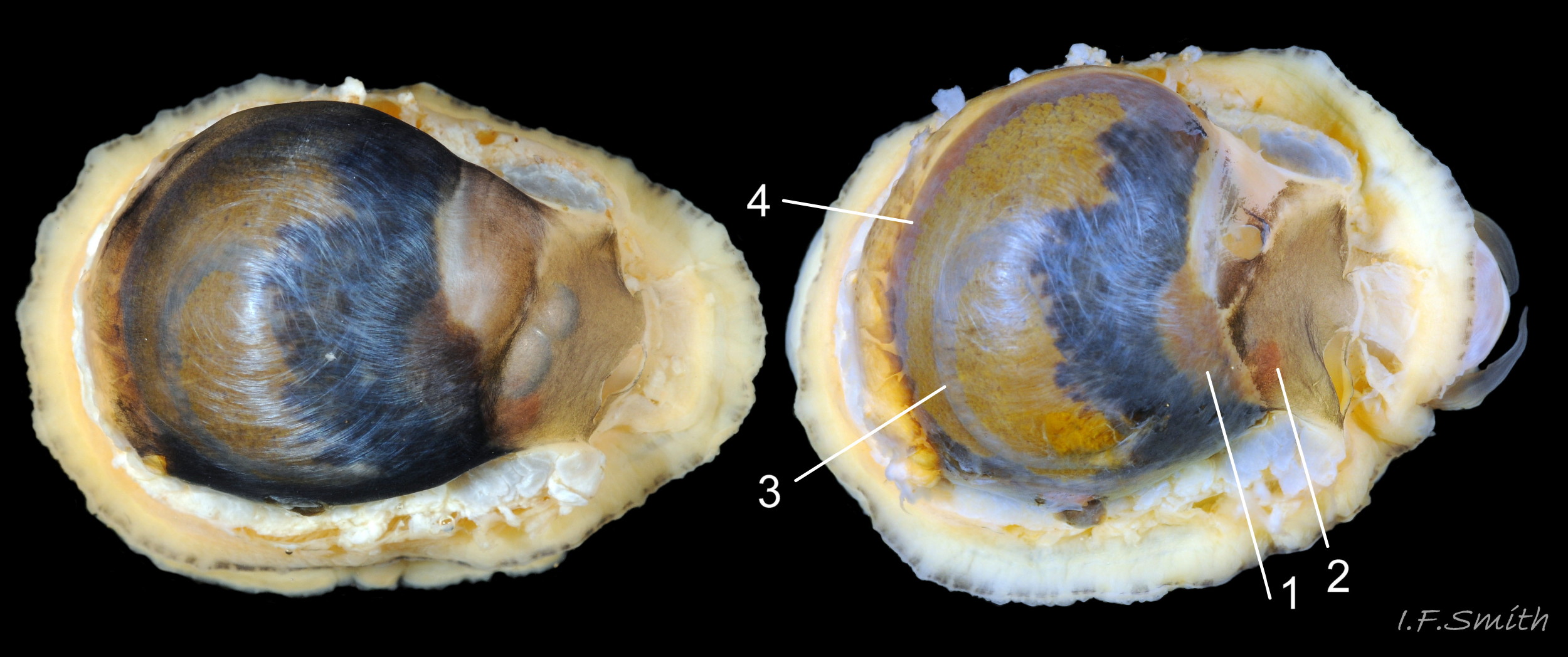
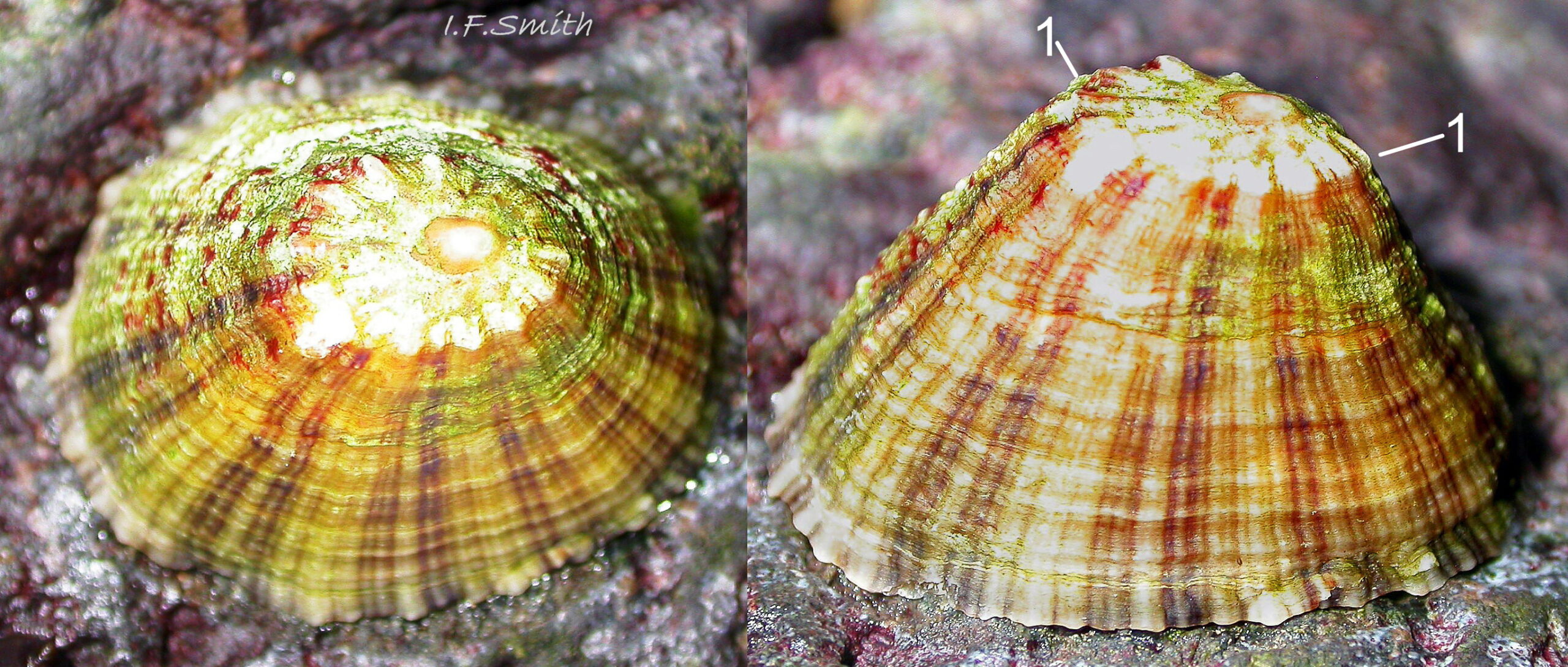
Maximum dimensions L. 50 mm, W. 40 mm and H. 20 mm (Graham, 1988). The strong shell is a conoid of widely varying proportions from very high, H/L c. 70% 01Pv & 02Pv to very low, H/L c. 20% 03Pv . On 15 specimens 7 to 46 mm long from eight sites in Wales and south, north-west and north-east England, many had the apex positioned anteriorly, as on P. ulyssiponensis and P. depressa, at about 36% shell length from the anterior 04Pv The range was from 31% 05Pv to 43% 02Pv . Only those at the upper end of the range had apices noticeably nearer the centre (50%) than the other species. The oval base is usually rounded at both ends and often narrower at the anterior 06Pv , but some have a straight posterior 14Pv resembling the description of P. ulyssiponensis by Graham (1988) and other authors. In profile, the sides of the conoid can be straight, concave or convex to varying degrees. The exterior has concentric growth lines and radiating ribs which vary from weak and inconspicuous 08Pv to strong and prominent when they can create small raised nodules where they cross 07Pv . The ribs are often eroded 01Pv , 09Pv & 10Pv or covered by epizoic growths 11Pv & 12Pv . In the grooves between the pale ribs there are often two or more radiating, fine, dark lines 13Pv which may merge into a broad band 14Pv .
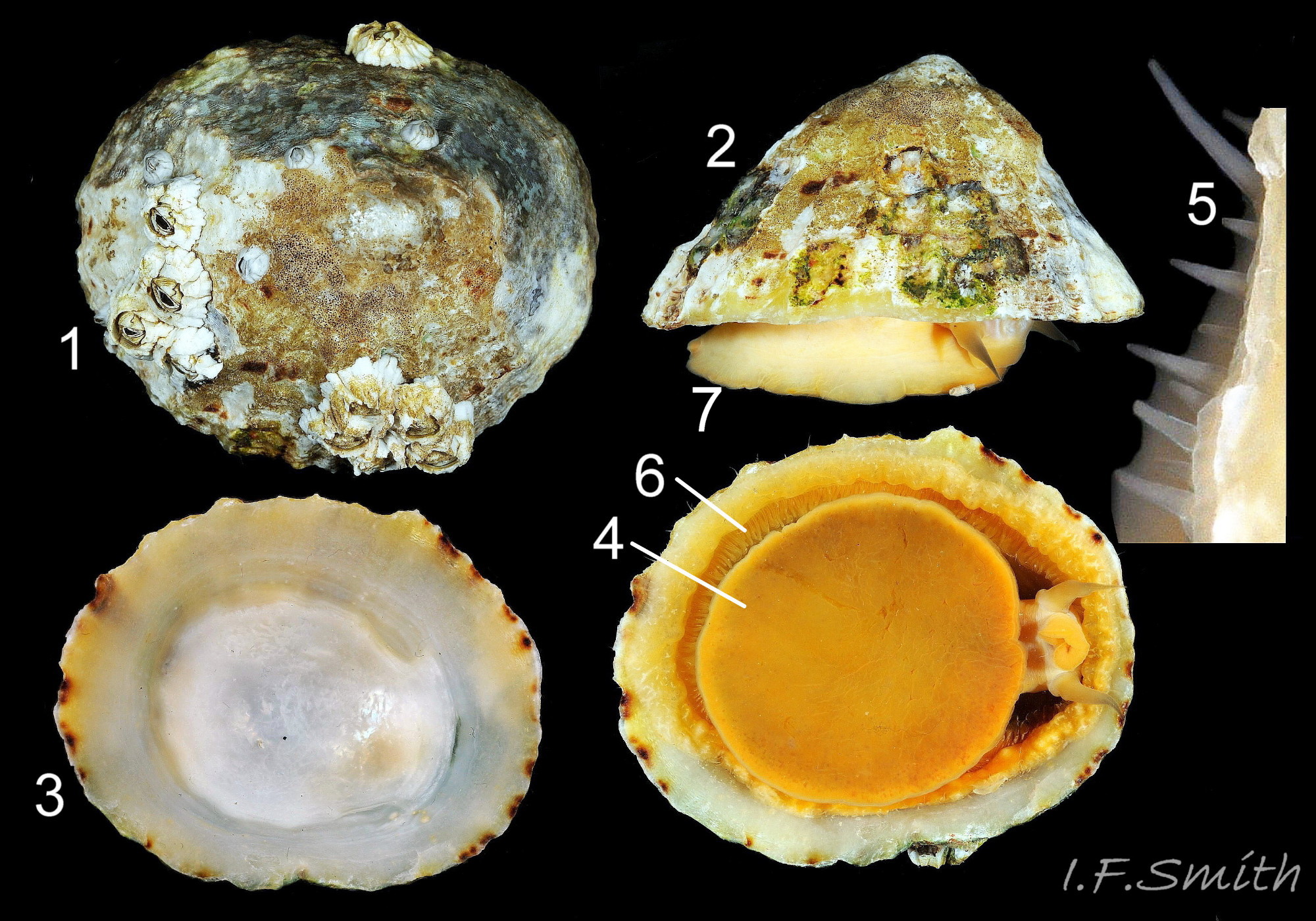
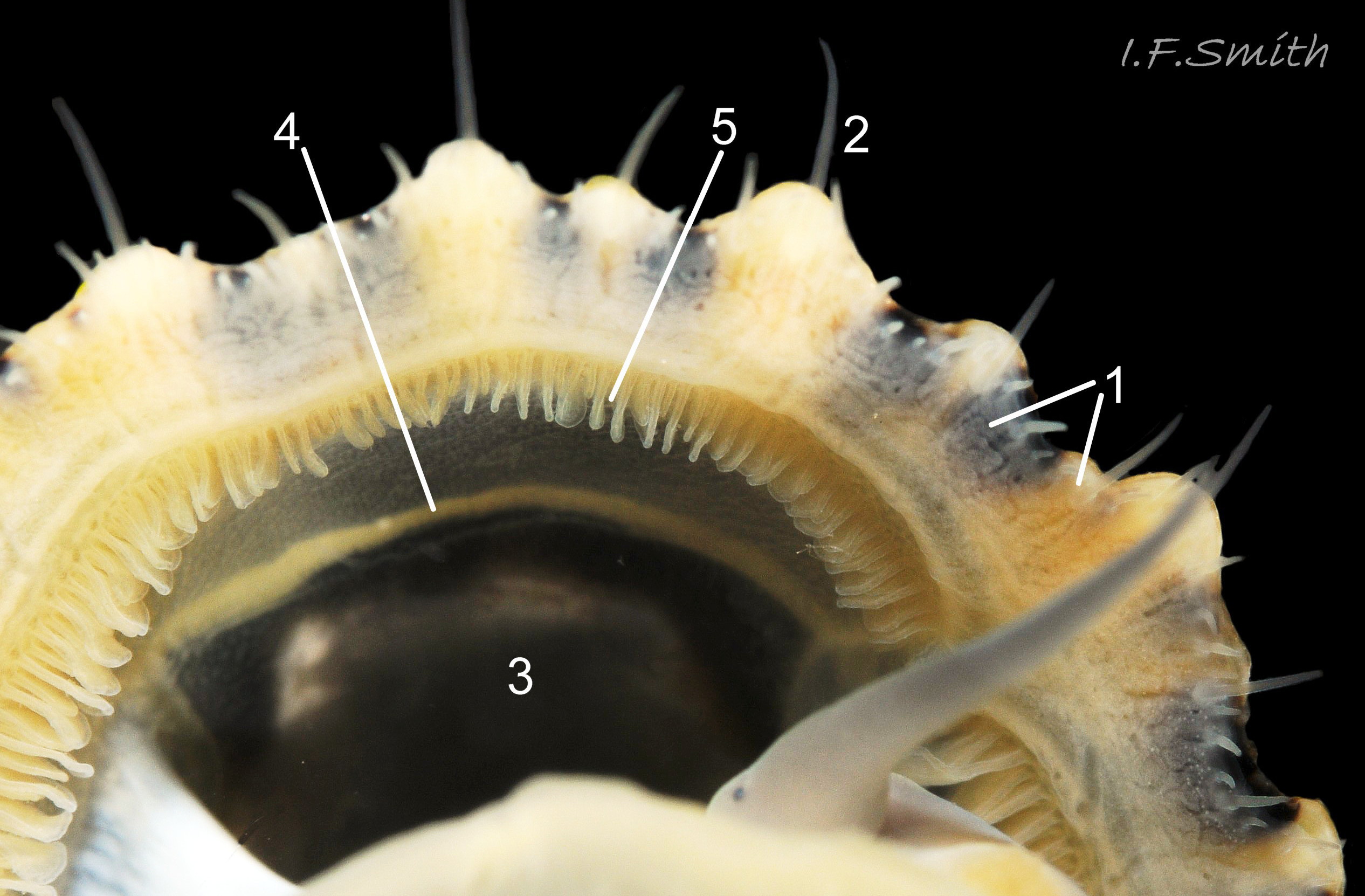
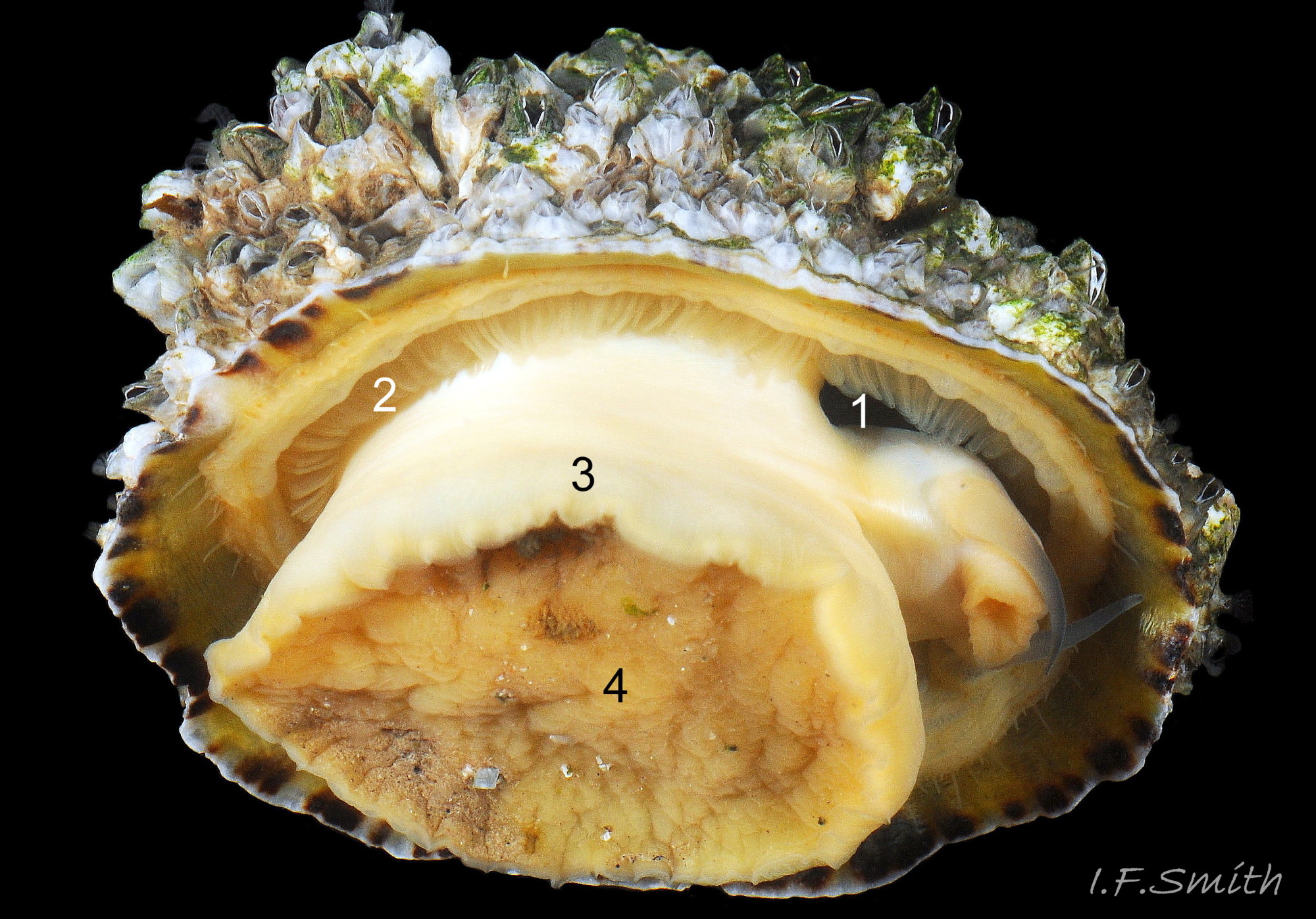
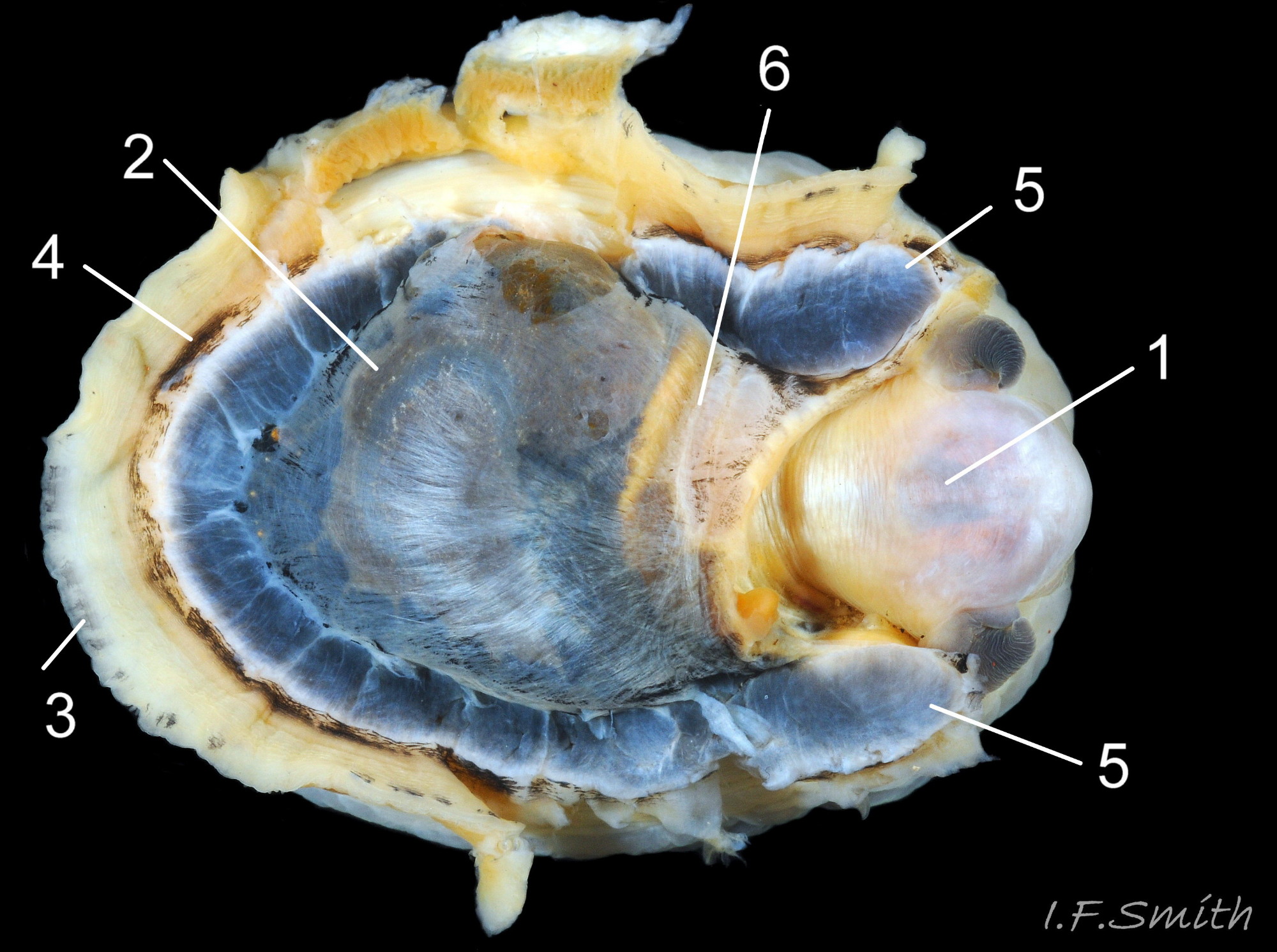
On the interior of the shell, the pedal-retractor muscle leaves a translucent horseshoe-shape scar called the ‘myostracum’, the mouth of which is closed by a thin anterior mantle-attachment scar 15Pv . The two scars enclose an area shaped like an amphora. The colours of the exterior pale ribs and dark radiating lines or bands are usually clearly visible on the interior through the transparent, iridescent layer 16Pv lying between the shell rim and the myostracum. The amphora area varies in colour; it is often orange when the limpet feeds on red algae 05Pv . Within the amphora, there is often a silver-grey or opaque white or grey vertex patch 06Pv . This is often inaccurately called the ‘head scar’; the head is located in the nuchal cavity forward of the amphora and is not attached to the shell, so leaves no attachment scar. The interior colours may get covered by an opaque white or cream porcelaneous layer on large specimens; it is probably deposited when growth ceases 17Pv & 06Pv .
Body description
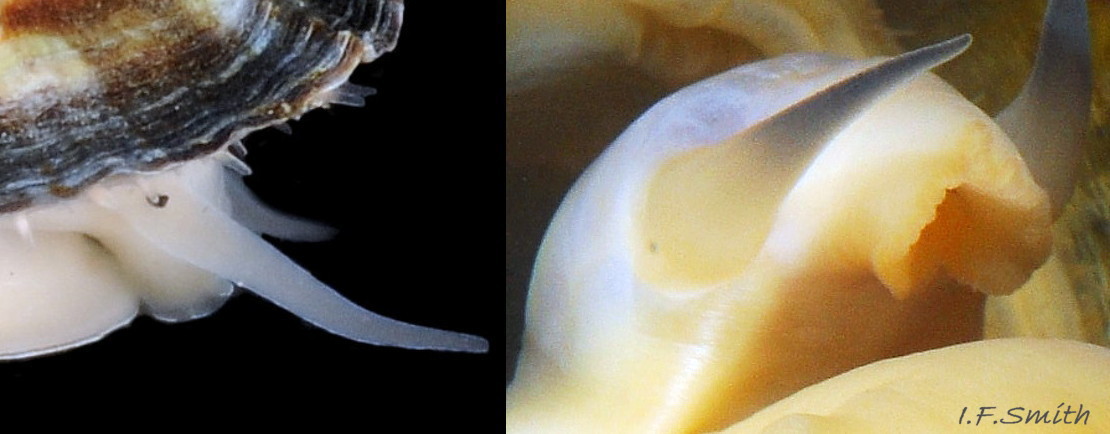
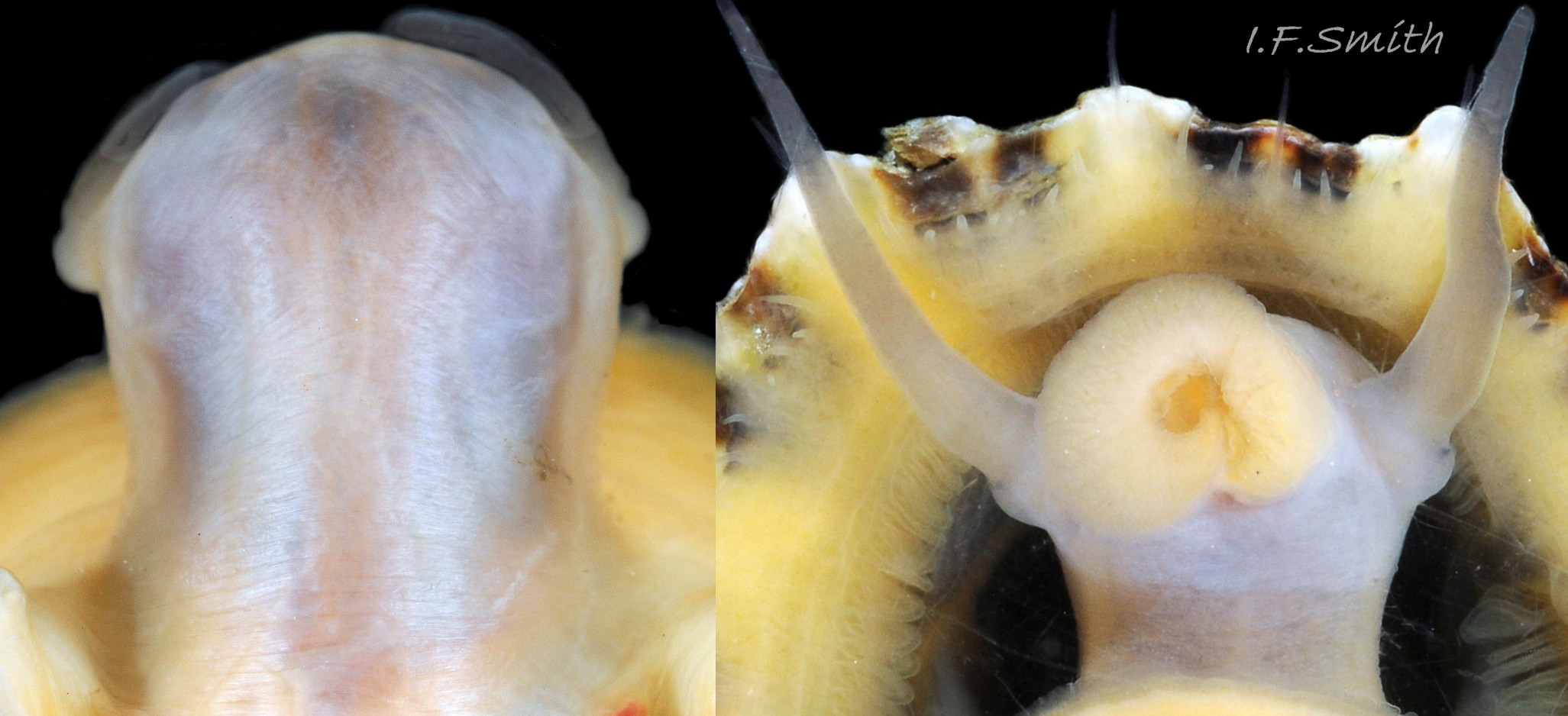
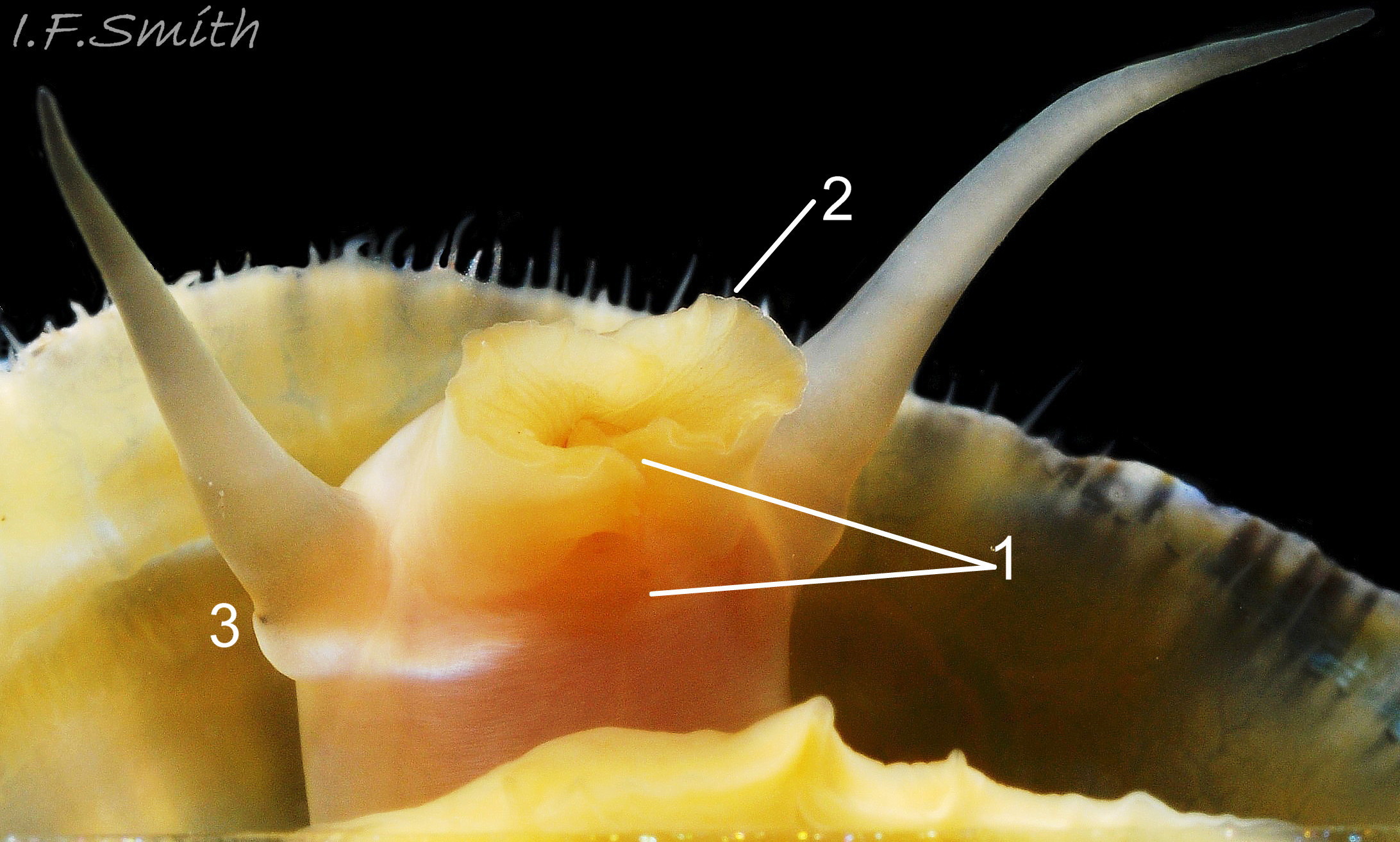
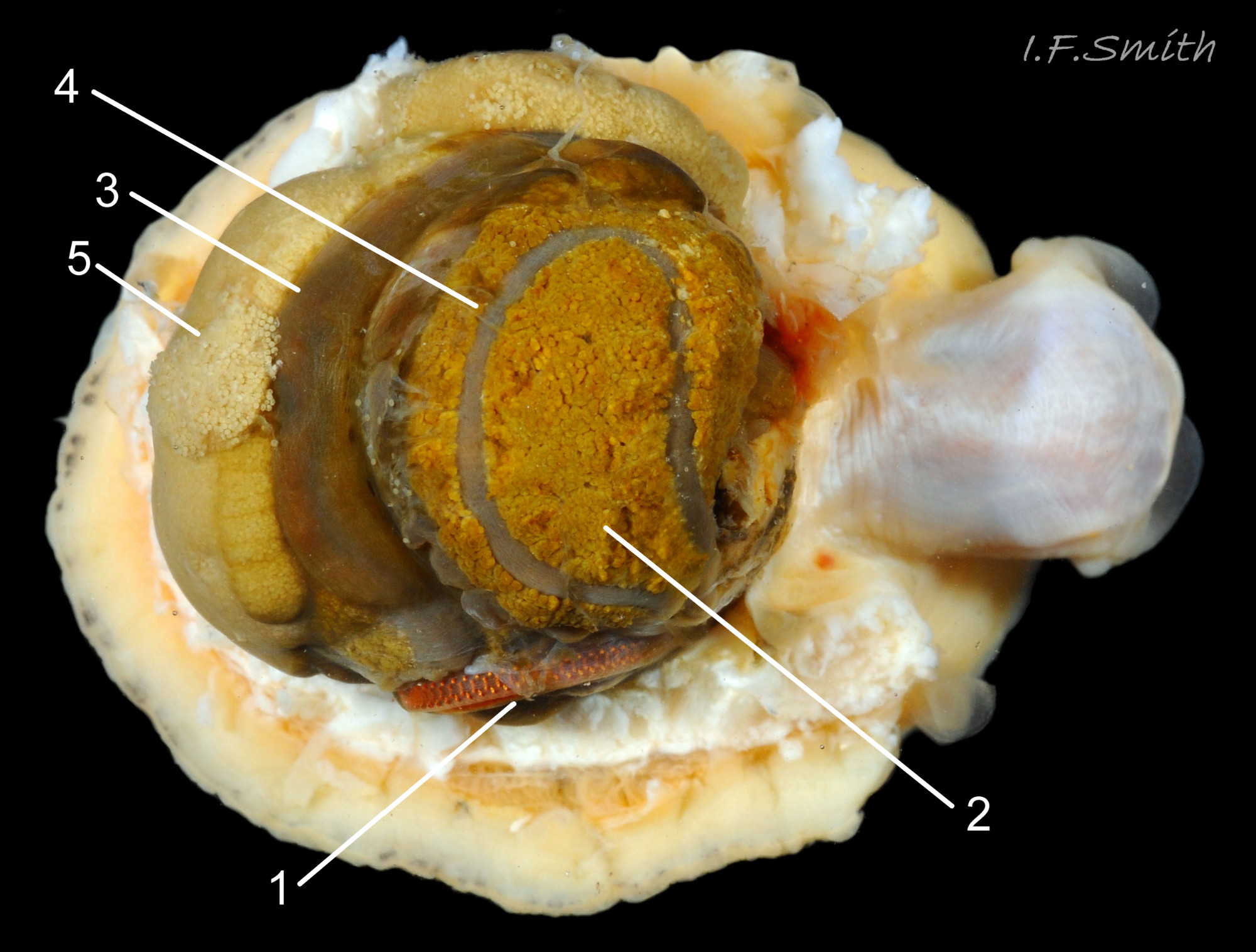
The translucent white head 18Pv shows the internal purple-pink odontophore in good lighting 19Pv . It has a substantial snout, slit at the posterior, with a large mouth, transverse when shut, fringed by thick outer lips 20Pv . The distal end of the snout and the lips on adults are often yellow. When the outer lips open, the darker yellow inner lips are exposed 21Pv. The inner lips open laterally 22Pv to expose the radular teeth, often yellow and damaged on the front three active rows, followed by red teeth ready to replace them. There is a cuticularized, triangular licker at the end of the radula.
The sturdy pale grey cephalic tentacles have a slight dorsal groove 23Pv and a small black eye on the edge of a raised collar at the base 20Pv . The eye is a primitive or degenerate cavity open to seawater and lined with black retina cells.
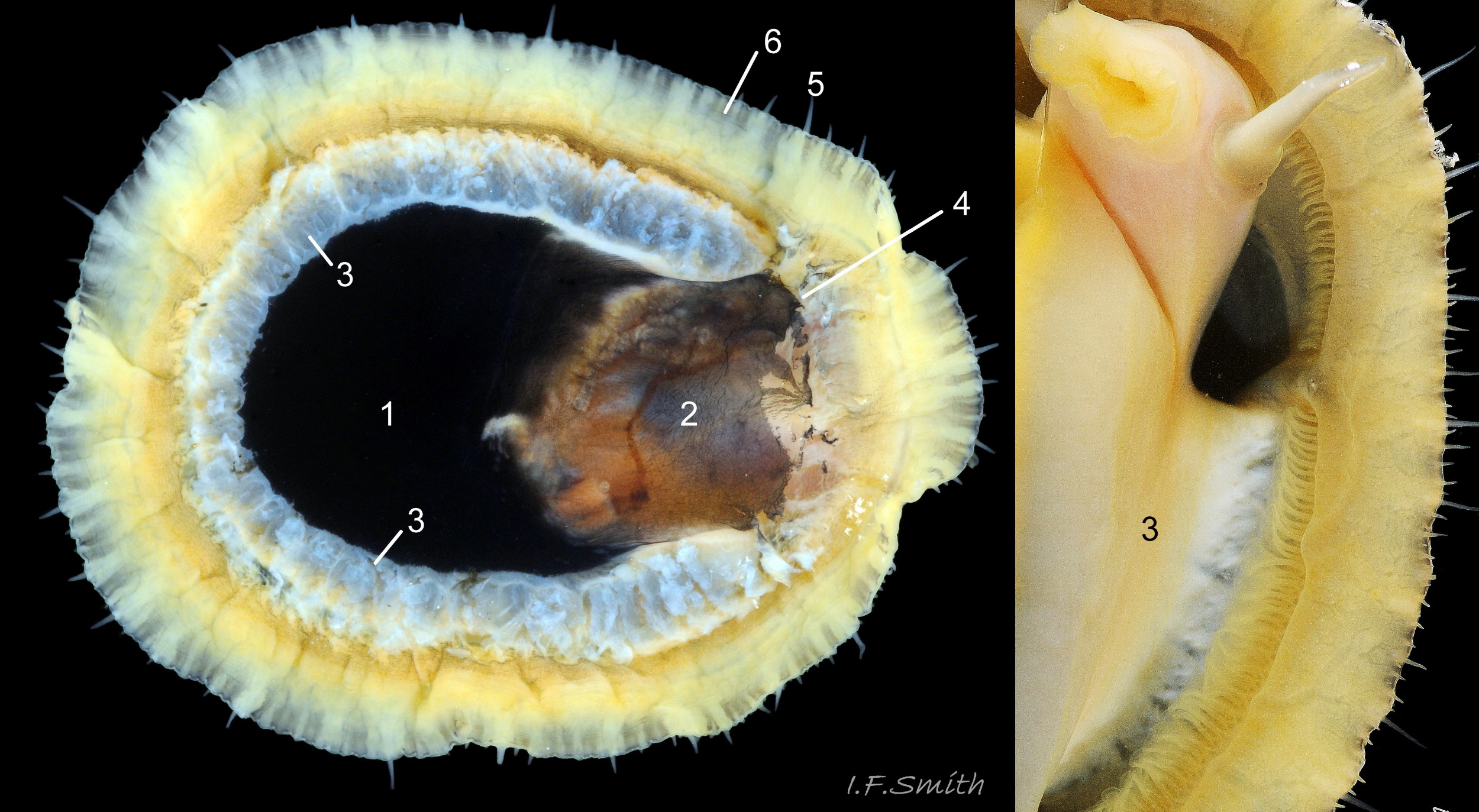
The pallial tentacles and mantle-skirt are translucent pale buff or whitish, but the skirt shows the colours of the shell through it 24Pv. The translucent tentacles are best viewed when expanded in water against a black background; they can be almost invisible against pale shell 25Pv .The mantle roofing the nuchal cavity over the head is black and/or blackish shades of other colours, and the mantle covering the visceral hump is covered with black pigment 26Pv which can be rubbed off leaving the mantle translucent .
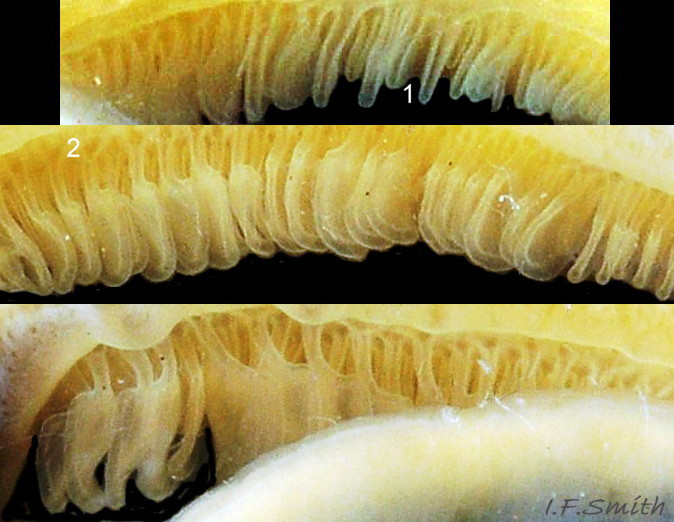
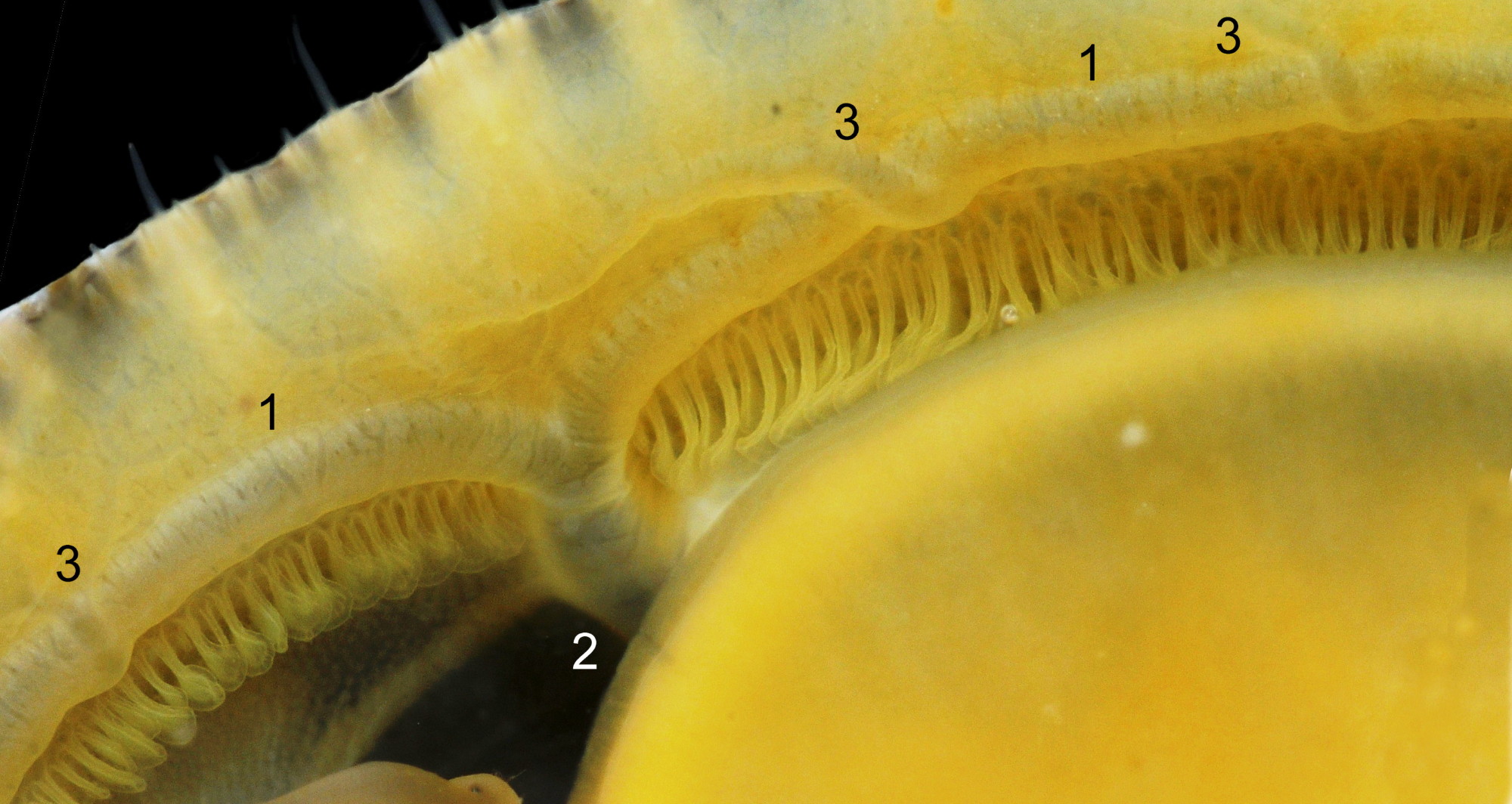
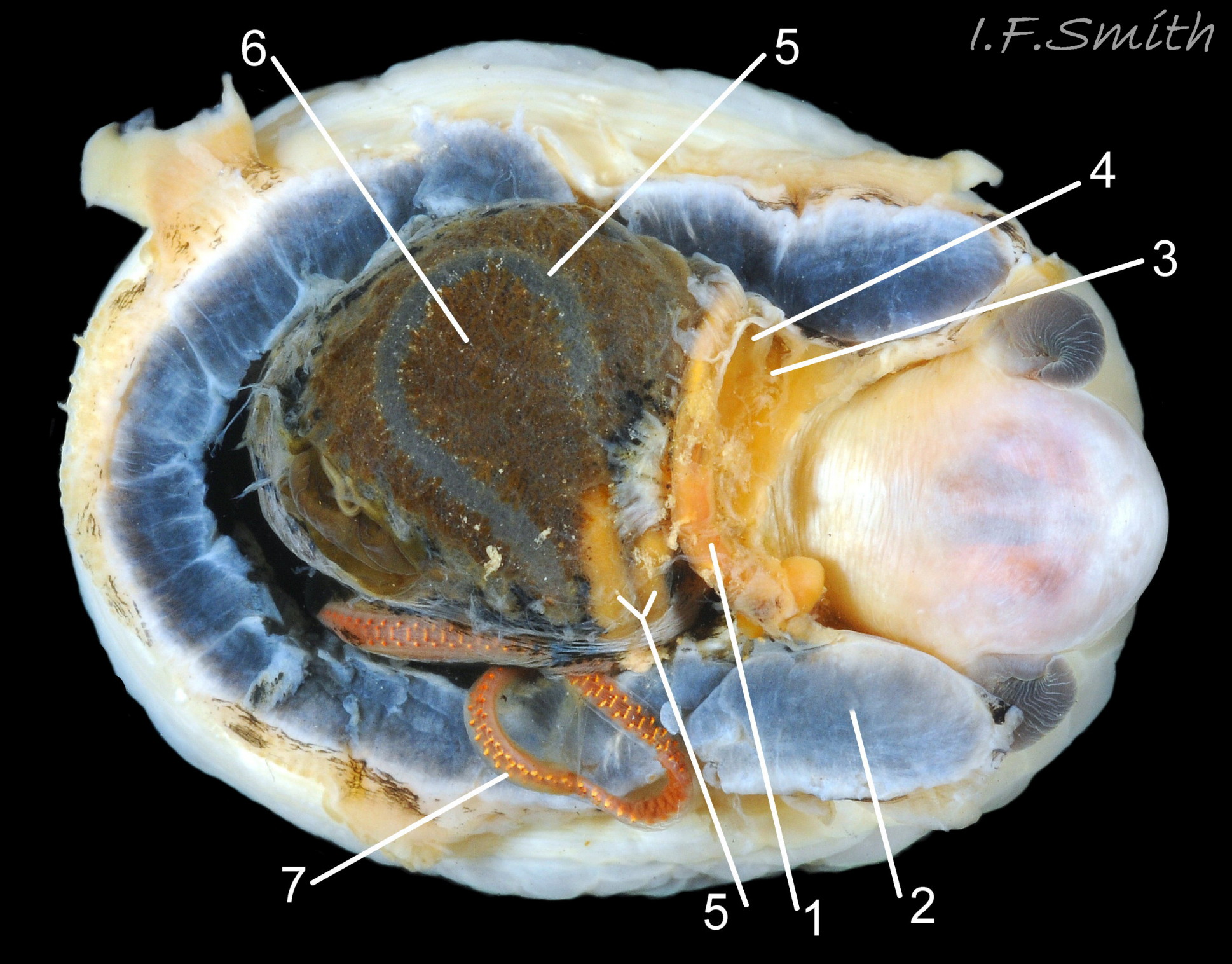
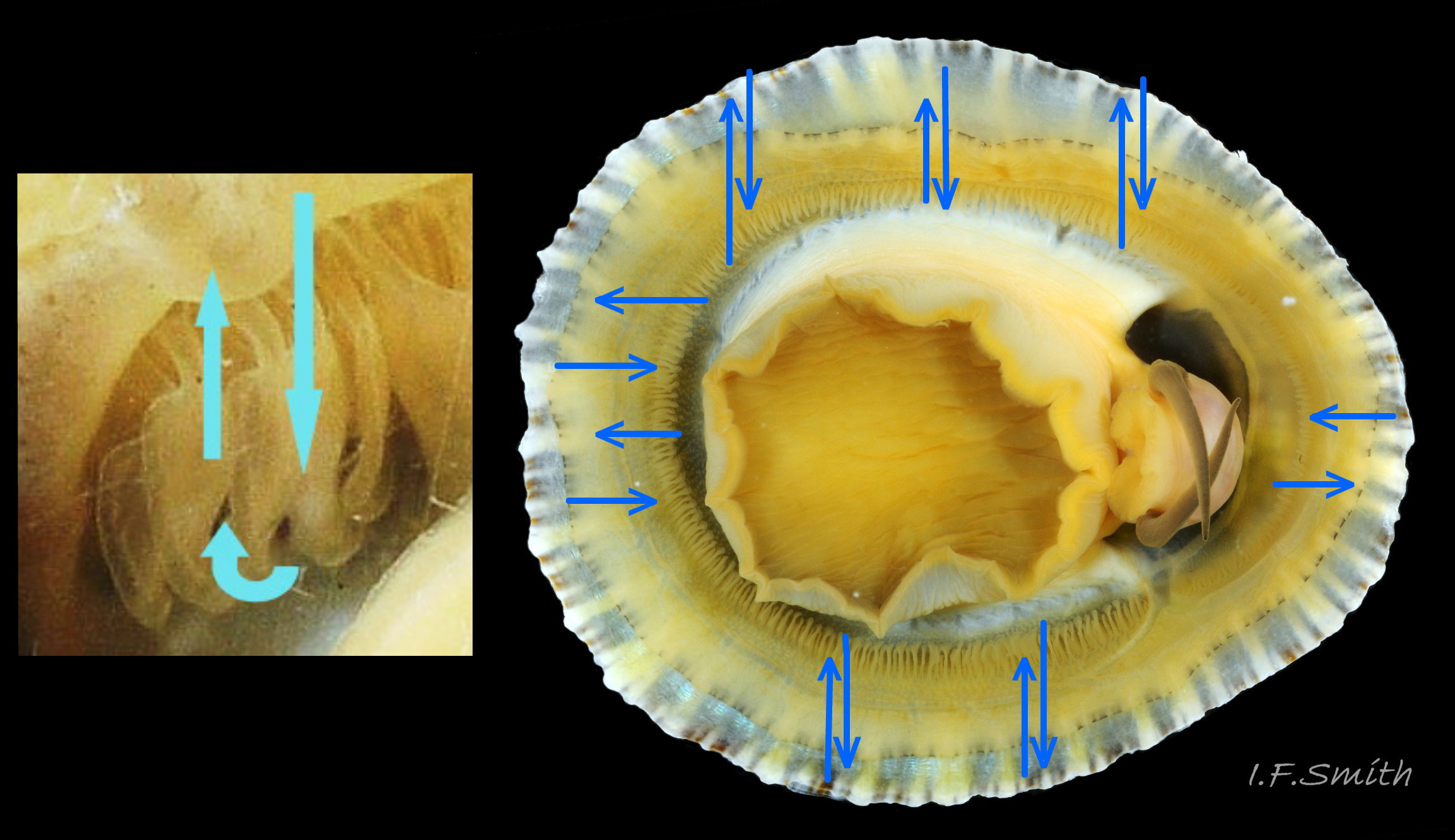
The mantle cavity consists of the nuchal cavity and a pallial groove filled with pallial gills around the entire periphery of the foot-head 27Pv ; there is no ctenidium. Each gill is a tongue-shaped leaflet attached by a stalk to the distal wall of the pallial-groove and has a densely ciliated groove on the stalk and thickened rim 28Pv .
The body is attached to the shell by the pedal-retractor muscle which consists of a horseshoe pattern of muscle bundles demarcated by gaps 26Pv & 29Pv . Next to the muscle distally, the afferent branchial vessel is usually mostly concealed by the pallial tentacles. Distally of the gills, the efferent branchial vessel encircles the whole body 29Pv and passes through a large trunk into the left side of the nuchal cavity 29Pv & 30Pv .
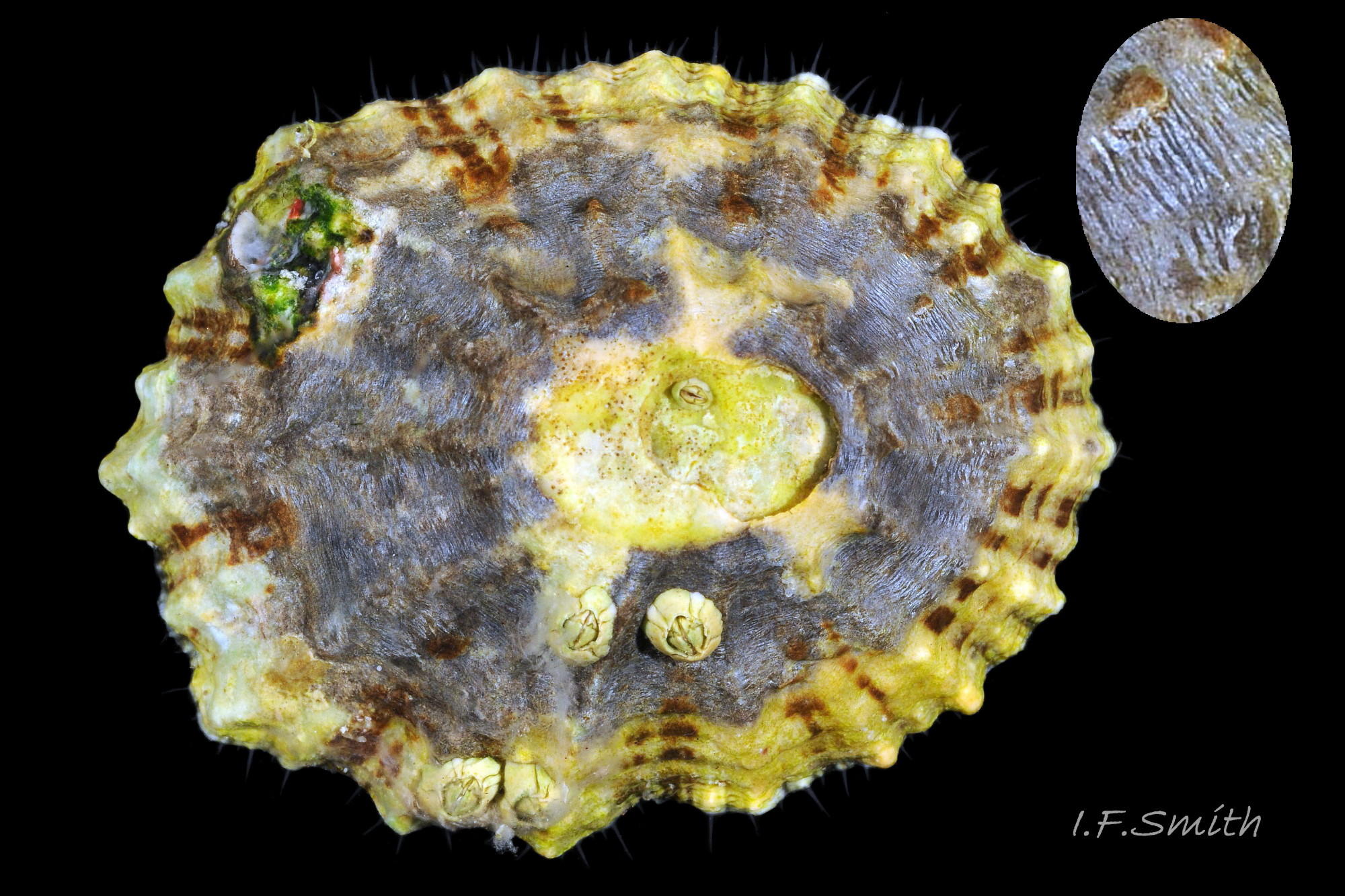
The approximately circular sole of the foot can be black, grey, khaki, orange, yellowish or whitish with a pale peripheral rim 27Pv & 31Pv . The sole is slightly translucent so its colour is affected by that of the gonads above it. When gonads are undeveloped, the dark viscera show as grey or blackish. Translucency revealing dark viscera is greater when in water than when in air 32Pv . The sides of the foot are usually white when young 33Pv becoming slightly yellowish or buff with age 11Pv & 12Pv . The foot lacks features such as epipodial tentacles. When crawling, usually only the extended pallial tentacles and, perhaps, tips of the cephalic tentacles protrude beyond the shelter of the shell 11Pv . There is no penis as fertilization is external.
Further detail visible with simple dissection
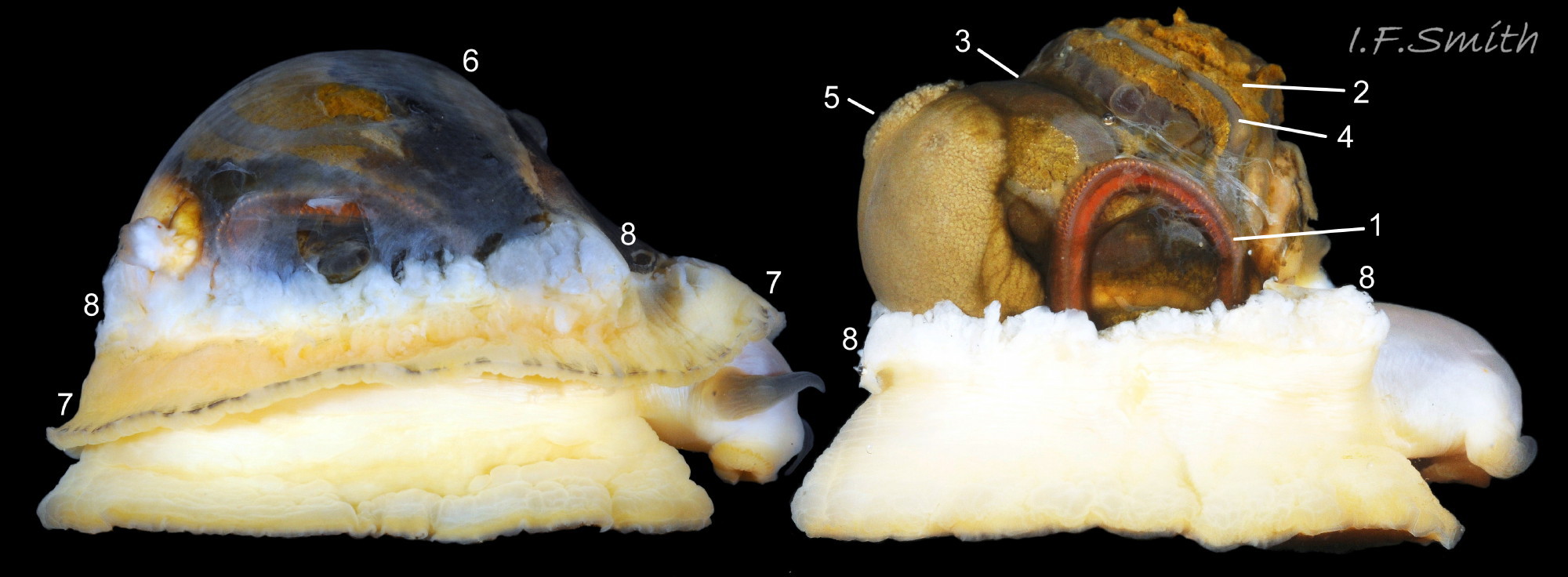
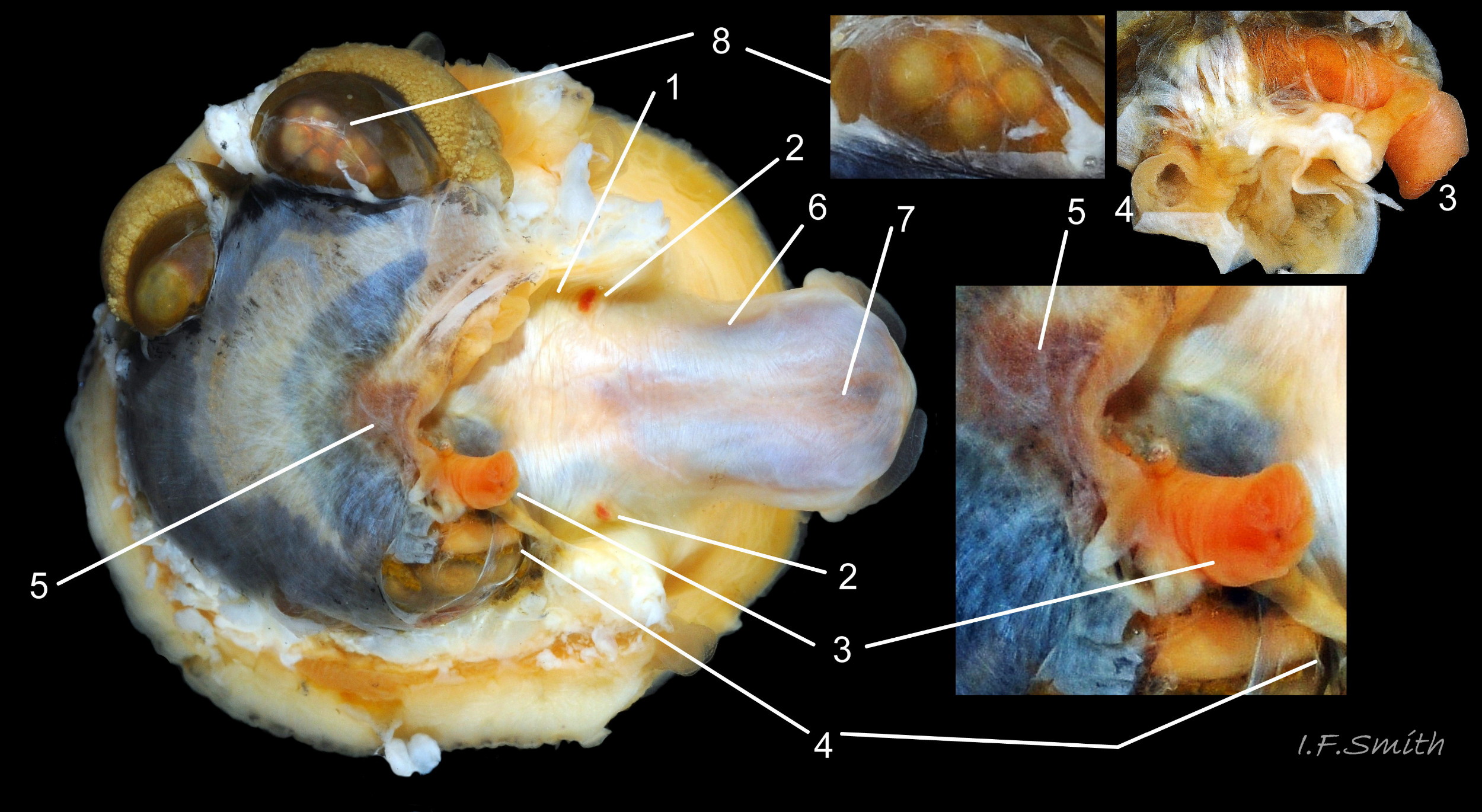
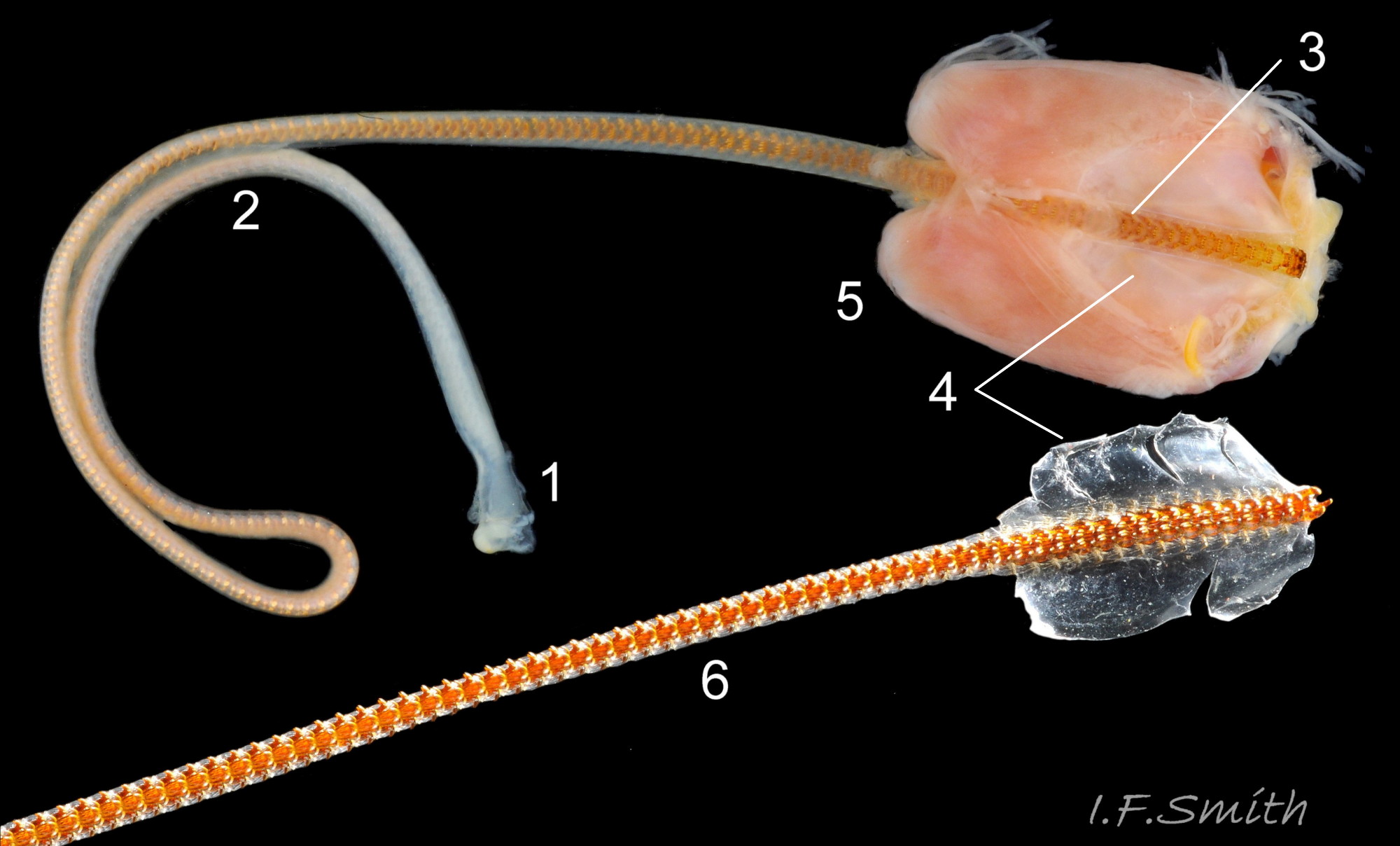
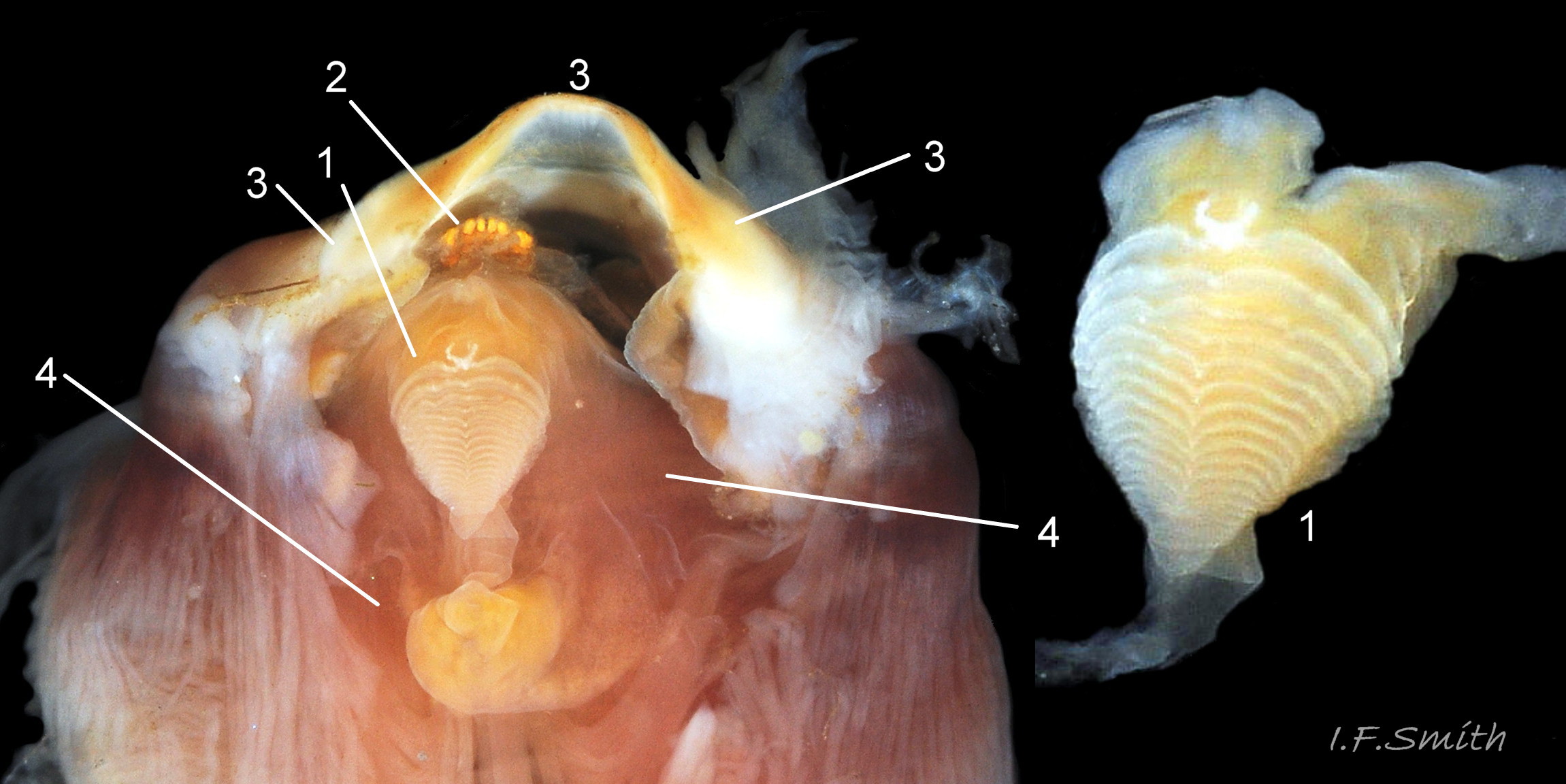
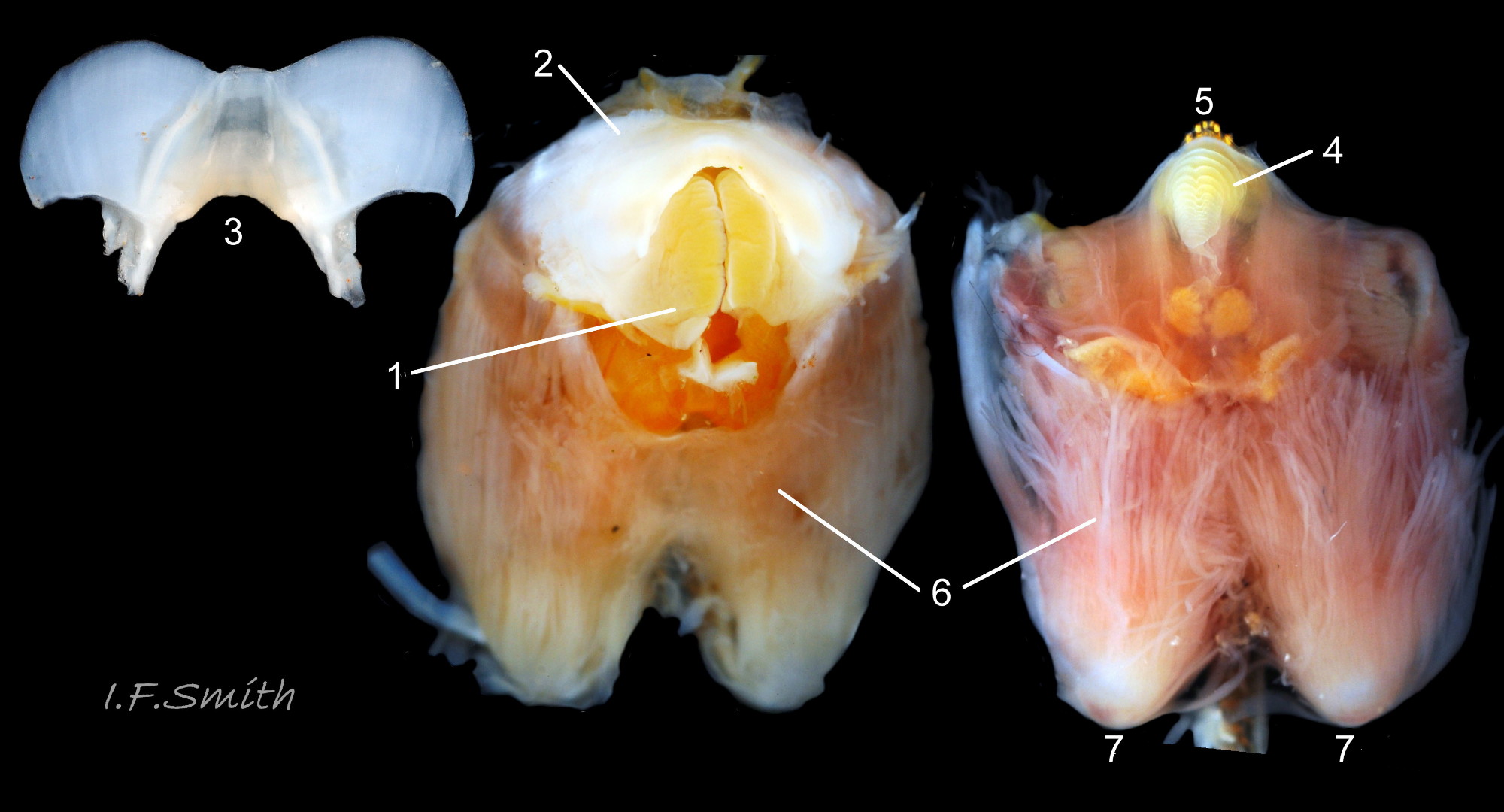
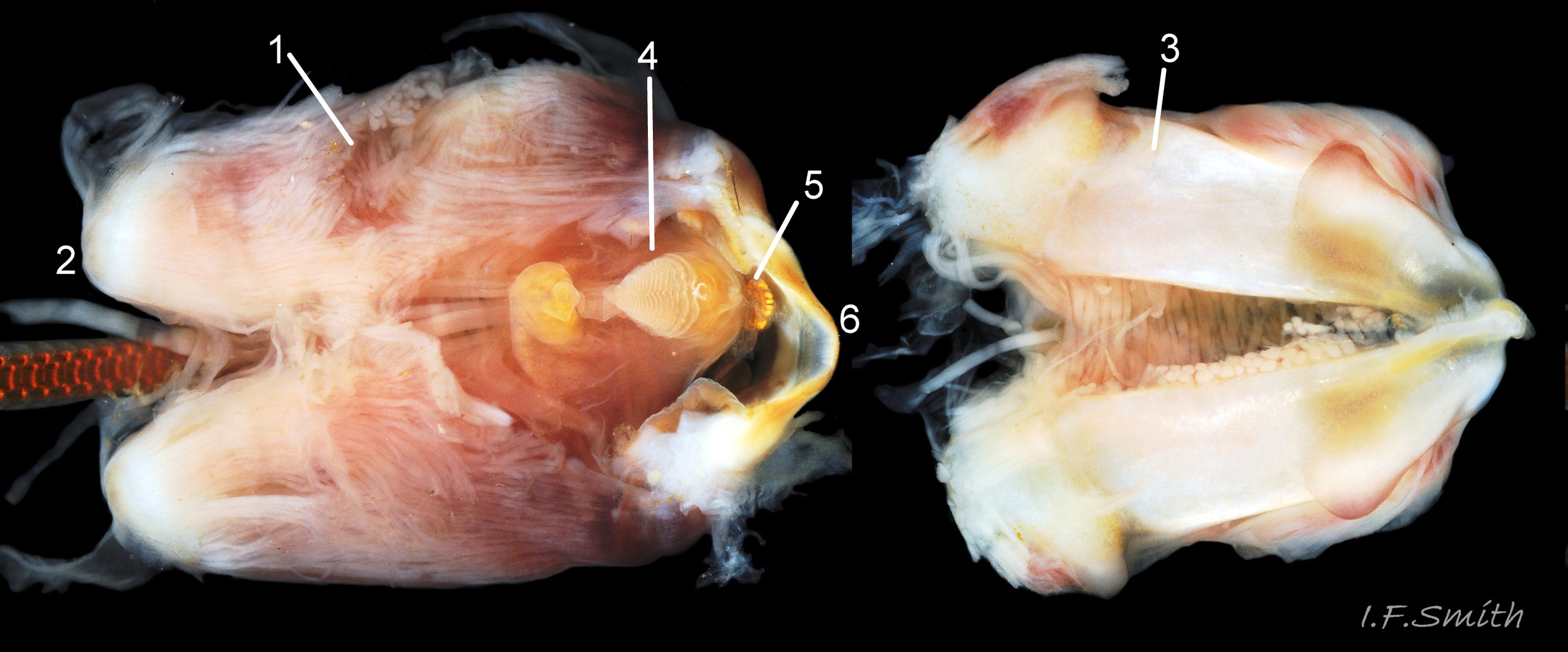
Shell removal by severing the pedal-retractor muscle shows the muscle-bundles clearly 26Pv . The anterior bundle on each side is the largest and strongest 34Pv & 38Pv as they must firmly pull down the shell further to the anterior where bundles are absent. Shell removal exposes the mantle subdivided into a pale translucent mantle-skirt, a narrow black band over the pallial-groove containing the gills 34Pv and a large black and dark pigmented amphora-shaped area over the viscera and nuchal cavity. Removal by rubbing of black pigment from the amphora area reveals the translucent mantle over the viscera 35Pv & 36Pv . When the translucent mantle is removed the viscera expand and there is a clear view of such organs as the radular sac, digestive gland, stomach, intestine, gonads 37Pv & 38Pv heart 34Pv & 38Pv left kidney, urinogenital opening of right kidney, rectum, anus and osphradium with associated neck warts 38Pv & 39Pv. Opening the head reveals the anterior of the radula with transparent hyaline shield resting on the twin bolsters of the odontophore 40Pv. Removal of the rest of the radulain its radular sac from the viscera shows it is longer than the body so is folded and coiled to fit inside 41Pv & 42Pv .
The radula is created in the radular sac. It starts translucent whitish at the posterior. As teeth travel forwards they acquire strong hard minerals of iron and silica from the sac, gradually increasing colour saturation 43Pv through shades of yellow and orange to almost red when ready for action in the front three rows at the anterior on the odontophore. Each row of teeth is arranged in the docoglossan formula, 3+D+2+R+2+D+3: a small, central inconspicuous unpigmented rachidian (R) flanked on each side by 2 large, unicuspid, pigmented lateral teeth, then a single, tricuspid, pigmented, dominant-marginal tooth (D) and three small unicuspid marginal teeth on each side of the radula 43Pv . A white, cuticularized, conical licker at the tip of the radula is divided into plate-like ridges by deep transverse grooves 44Pv . A white, chitinous, unarticulated ‘jaw’ (3) is attached to the anterior of the odontophore where it is arched to frame and protect the inner lips of the mouth 44Pv & 45Pv . The bolsters of the odontophore are made of firm white cartilage 46Pv . Gonads develop initially between the foot and viscera above, later spreading up around the visceral mass 47Pv & 35Pv.
Identification of patellid limpets.
P. vulgata has wide ranges of shell colour and form, including many of those found on P. depressa and P. ulyssiponensis. Printed identification guides are constrained by space to give short descriptions which omit many variations and sometimes contradict each other. The appendix below compares the descriptions in several marine mollusc identification guides.
Limpets are “very difficult to distinguish by shell alone” (Forbes & Hanley, 1849).
“Undoubtedly there are fundamental differences in the shell of the different species, but only the most experienced worker can separate these from the abundant variations presumably of environmental origin.” (Fretter & Graham, 1962)
Defaulting uncertain identifications to ‘P. vulgata‘ because ‘vulgata‘ means ‘common’ has lead to multiple misidentifications and corruption of distribution maps. It is not the commonest limpet in much of south-west England and further south in Europe, and it is absent from the Mediterranean where GBIF has multiple erroneous records of it.
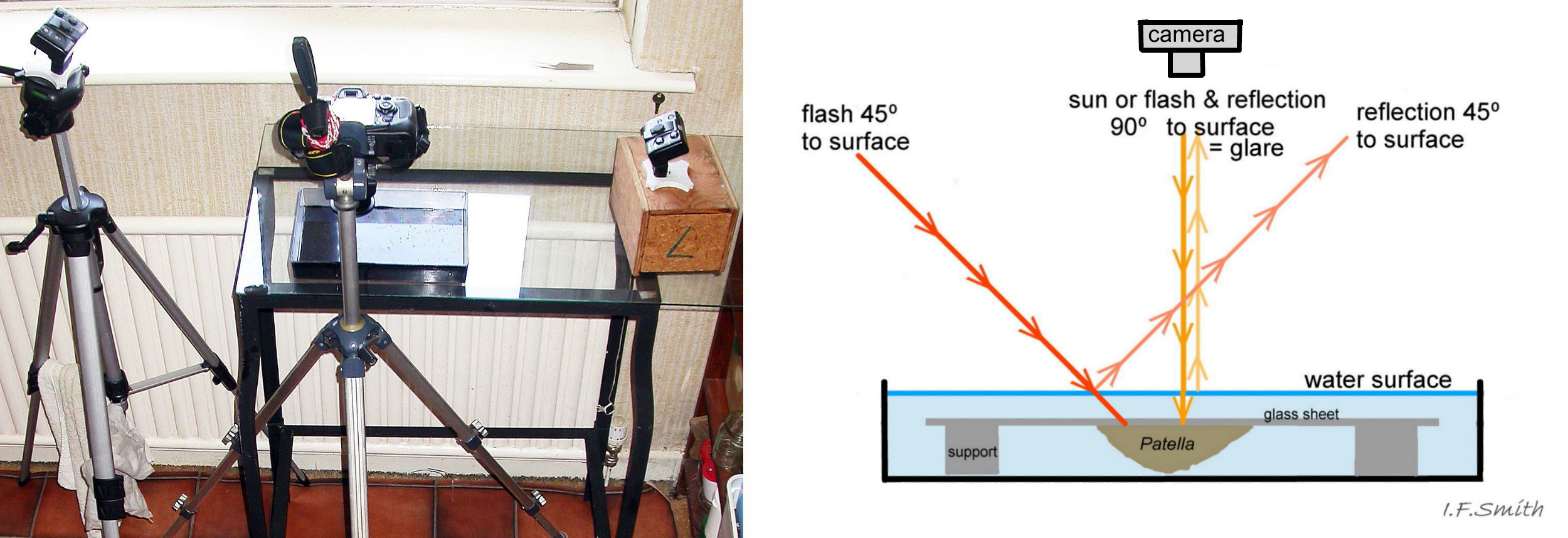
Relying on shell exteriors alone will lead to misidentification. The interior of fresh shells, not worn or bleached specimens, will help in many cases 06Pv & 17Pv , but examination of the colours of both the sole and extended pallial tentacles 48Pv is needed for consistent accurate discrimination of the three rock-dwelling Patella species of north-west Europe. This is best achieved with the specimen adhering to the underside of a supported glass-sheet in a black-based container of seawater 49Pv . If in air, the pallial tentacles may be withdrawn or may reflect glare which gives a false impression of opaque white 50Pv .
Key identification features.
Patella vulgata
Concise essential diagnostic features: translucent pigmentless pallial tentacles, with any colour of foot. All round Britain and Ireland where suitable bedrock, boulders or stable shingle; dominant species of Patella except in south-west England and some exposed sites near low water mark.
Shell exterior
1) When height is 55% or more of length 01Pv & 02Pv , it is usually diagnostic of P. vulgata in north-west Europe, but it varies from c. 70% to as low as c. 20% of length 03Pv & 04Pv . If less than 55%, specimen can be P. vulgata, P. ulyssiponensis or P. depressa. Photographs need to be direct lateral view, not oblique. No other exterior shell feature is reliably diagnostic. Features such as sculpture, aperture outline and centrality of apex vary and overlap with those of P. ulyssiponensis and P. depressa.
Shell interior
2) Silver-grey or opaque white or grey patch on vertex 06Pv within amphora shaped area is diagnostic when present, but some P. vulgata lack a vertex patch. Colours of other parts of interior vary and can occur on P. ulyssiponensis or P. depressa.
Soft parts
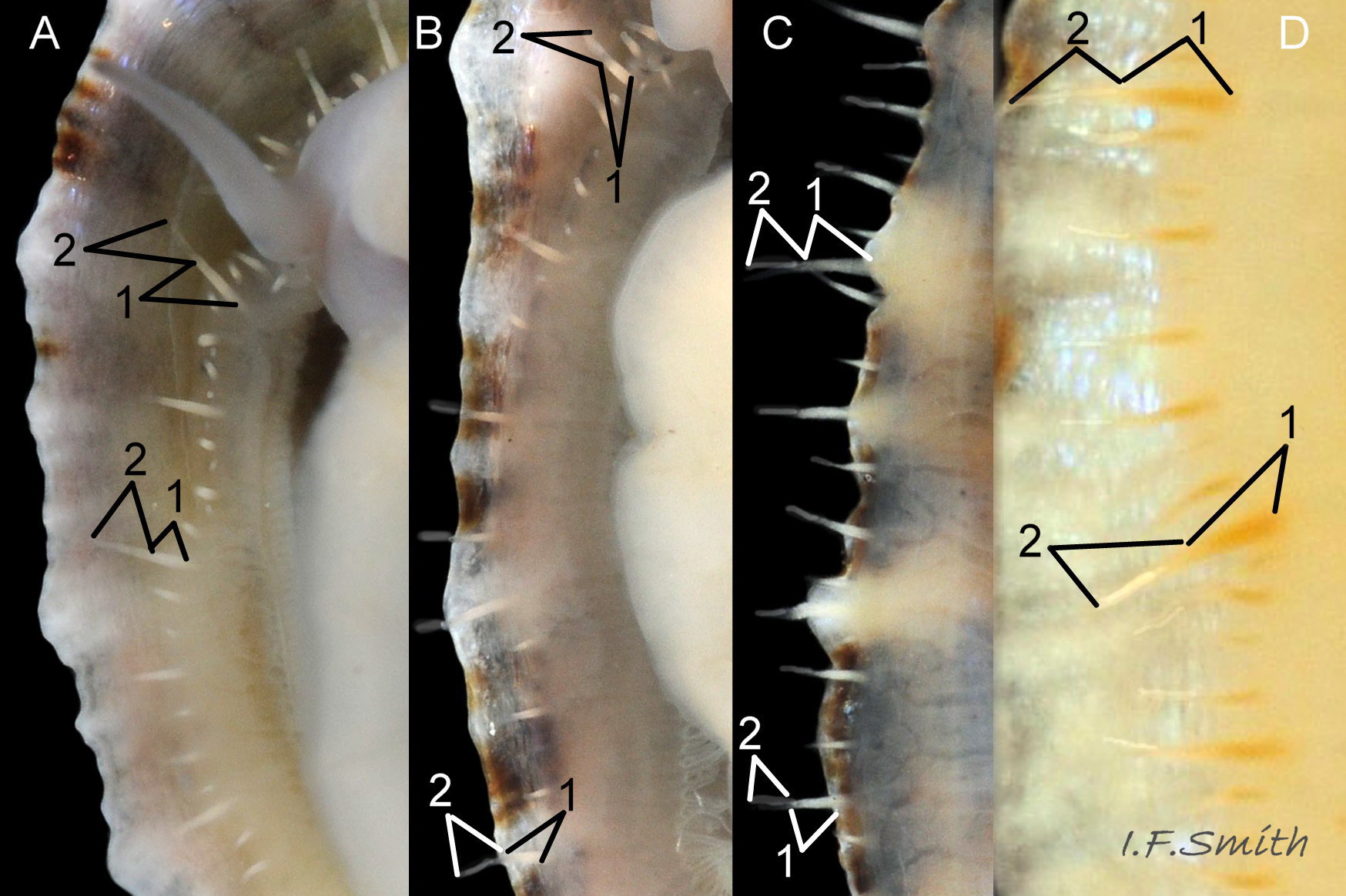
3) Diagnostic pallial tentacles on perimeter of mantle are off-white translucent with no opaque white or yellow pigment when fully extended 25Pv , 48Pv & 49Pv.
Similar species
Patella depressa
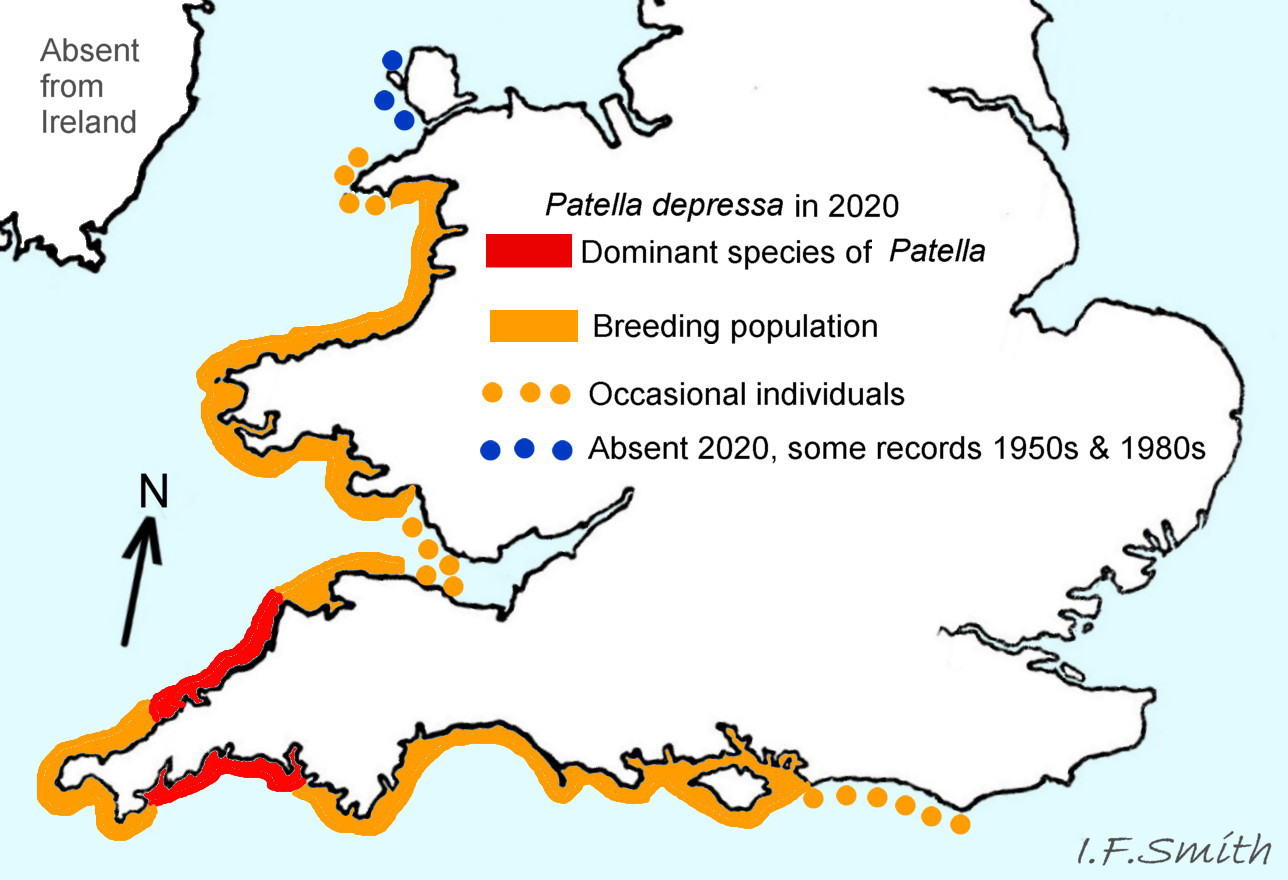
Concise essential diagnostic features: opaque, chalk white pallial tentacles combined with black or blackish sole. Be aware of reflected glare on translucent pallial tentacles of P. vulgata in air 50Pv . The 2020 UK distribution was south coast England to Llŷn Peninsula Wales 51Pv. Dominant Patella species on many shores in south-west England and Brittany (Kendall et al. 2004, Oróstica et al., 2020) . Not in Ireland. On bedrock in pools, seawater trickles or other damp positions; not on shingle or boulders.
Shell exterior
1) Low shell 52Pv , H/L 25-37% in sample of twelve typical shells from S.W. England and N.W. Wales (rare exception to 54%), is not diagnostic as these heights also occur in P. vulgata. Features such as sculpture, aperture outline and centrality of apex vary and overlap with those of some P. vulgata.
Shell interior
2) Amphora area often white with grey showing through it and a yellow periphery. Pallial gill groove scar, distal of translucent myostracum, is usually opaque white 53Pv . Whitish projecting points of ribs often have short, unglazed, chalky, pure-white central line, but reduced or lacking where projecting points of ribs eroded.
Soft parts
3) Pallial tentacles are opaque chalky-white for more than half of extended-length; may have small translucent tip; distinctly whiter than buff mantle-skirt from which they arise. Sole of foot pitch-brown to black 54Pv .
Patella ulyssiponensis
Concise essential diagnostic features: opaque white, sometimes yellow/orange, pigment on basal half of pallial tentacles combined with whitish, yellowish or apricot sole. Important that tentacles fully extended in water as translucent distal half could lead to misidentification as P. vulgata. At exposed sites all round Britain, except Flamborough Head to Kent and north-east Irish Sea, near low water mark and at higher levels in rock pools lined with coralline.
Shell exterior
1) Low shell 55Pv ; not diagnostic as some P. vulgata have equally low shells. Features such as sculpture, aperture outline and centrality of apex vary and overlap with those of some P. vulgata..
Shell interior
2) No whitish-silver-grey patch on vertex. 55Pv .
Soft parts
3) Basal half of fully extended pallial tentacles 56Pv have opaque white, sometimes yellow/orange, pigment; distal-half fades to translucent. Opaque basal parts often distinct from translucent mantle-skirt that they arise from, but not as distinctly chalk white as on P. depressa. Sole of foot cream, yellowish to apricot on adults 57Pv, white, sometimes with darkish shadow of viscera, on juveniles 58Pv .
Habits and ecology
P. vulgata occurs, often abundantly to 300/m², on British and Irish shores from the most sheltered to the most exposed, wherever there are bedrock, boulders, stable stones or hard man-made structures to which it can attach on their upper surfaces. They occur in salinities down to 25‰ and can withstand intervals down to 3‰ (Fretter & Graham, 1976). The shore zones it occupies between MHWS and ELWS and time it can endure exposure to air, 5% to 90%, vary with local conditions (Fretter & Graham, 1976). The damp cover of algae or clumps of Mytilus edulis can enable it to live at higher levels where long emersion would be otherwise too desiccating. Shade from rock and spray on exposed shores sometimes enable P. vulgata to extend up to EHWS (fig. 56 in Lewis, 1964). The lower limit at ELWS varies less except where hard surfaces are unavailable on the lower shore, and sometimes on exposed shores it is out competed by P. ulyssiponensis . In estuaries, sediment coating hard surfaces and turbidity preventing growth of its algal food other than near high water mark can limit P. vulgata to that high zone 59Pv .
Young specimens favour pools and damp conditions on the lower shore where there is little necessity to clamp down against desiccation during emersion. On them, and any adults remaining in those conditions, the relaxed mantle creates a shell with a low profile similar to that of P. ulyssiponensis and P. depressa. Some individuals move higher up the shore into drier conditions, even bare well drained rock 09Pv . They survive desiccation by clamping down hard on the rock during emersion. The contracted muscles pull in the mantle which results in the creation of a more narrowly based tall shell 17Pv & 60Pv .Some shells have an abrupt change of slope marking when a flat juvenile moved to drier conditions and developed a steeper profile 61Pv .There, in the absence of much competition, they often develop large, thick, high-domed shells. This is often most marked in the fluctuating salinity of estuaries where they may also need to clamp down at times while immersed to prevent contact with fresh water (Fretter & Graham, 1976) 02Pv .
P.vulgata feeds on the film of algal sporelings, diatoms and organic detritus coating rock surfaces (Yonge & Thompson, 1976), and mature fucoids and Ascophyllum are also rasped (Fretter & Graham,1976). Grazing is facilitated by powerful muscles on the large buccal mass 45Pv, and by the hard, iron-mineralized teeth on a long ribbon with plentiful replacements for worn teeth 41Pv . Radula length varies seasonally; it is shorter when wear of active feeding exceeds growth rate (Goshima et al., 2002)). Three or four rows of teeth are in contact with the substrate during feeding and up to two rows of teeth per day are worn out 22Pv . Loose particles are retained by the rim of the jaw surrounding the inner lips 45Pv and by the licker 44Pv which sweeps them up at the end of the radula stroke.
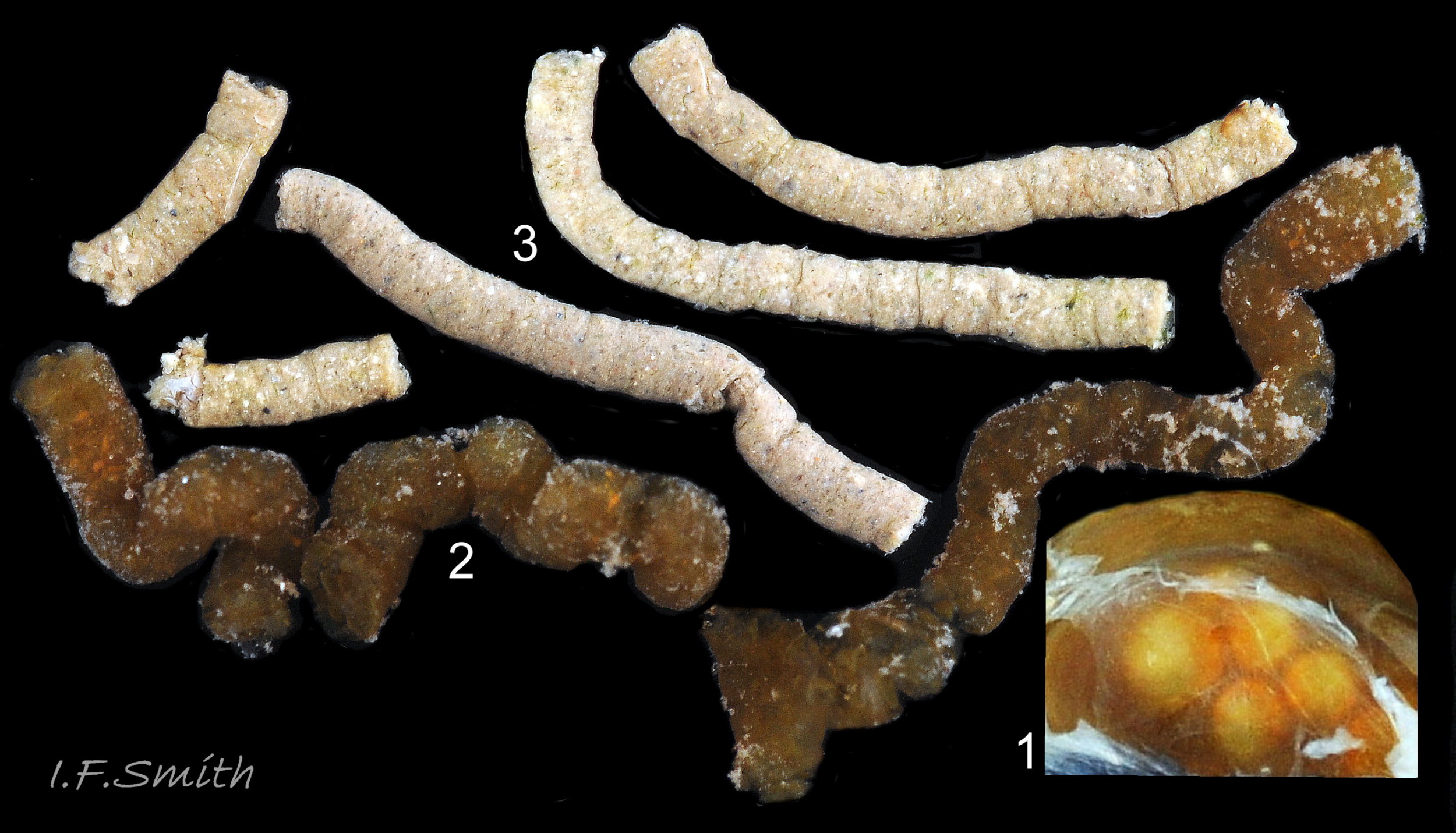
The algal film is stripped by the radula as the head sweeps left and right while the limpet slowly advances. On soft rocks the radula often leaves scratch mark in the form of a ribbon swinging left and right with closely spaced transverse marks left by each stroke of the radula 62Pv. Ingested food passes along the oesophagus to the extremely long intestine which, being eight times the length of the shell, loops six times in the visceral mass (Fretter & Graham, 1962) 38Pv. As the contents pass along the intestine the initially spherical contents are progressively compressed into faecal rods of closely adhering discs 63Pv which are expelled from the anus in the nuchal cavity 39Pv and transported by ciliary action to midway along the right pallial groove where they wait 32Pv for expulsion from the shell by periodic raising and abrupt lowering of the shell edge (Fretter & Graham 1962 & 1994). When P. vulgata is removed from rock, an accumulated pile of faecal strings is often found in position. The prolonged compression is needed to form firm faecal strings that will not contaminate gills in the pallial groove. This compensates for adults lacking a hypobranchial gland which produces mucus to bind particles.
Cilia create a water-current from left to right through the nuchal cavity of specimens less than 10 mm long, but the excreta and ova/sperm from the urogenital openings 39Pv in the nuchal cavity of adults are, like the faeces, carried by cilia to the exit point of the right pallial groove.
P. vulgata makes feeding excursions while immersed and in humid conditions while emersed, especially at night,. Adults return to their home scars probably by following back the mucus trail they left. There, the shell closely fits the surface because the limpet grinds the rim against the substrate to erode the rim to fit the contours of hard rock or to form a pit in soft rock 64Pv . There is often a raised central area in the pit where the foot attaches. The surrounding recessed area is cut by the shell rim as the limpet grows and the perimeter increases. The close fit enhances protection against predators, and against water-loss when emersed at low tide. The home scar is not permanent as within six months most P. vulgata have moved to a new position up to 30 metres away (Yonge & Thompson, 1976). A fight ensues when another limpet tries to take over the home scar with the opponents pushing each other with their shells. Also, the shell rim is used defensively with a chopping action on the tubular feet of predating starfish (Hawkins, 2013).
Grazing by P. vulgata is a major influence on the abundance of algae and the associated organisms on British and Irish shores. When all limpets and larger seaweeds were removed experimentally from a 10 metre wide 115 m long transect of shore in the Isle of Man in 1946-48 (Jones, 1948 and Yonge & Thompson, 1976) the rocks were very soon covered by a carpet of small green algae. This was followed by settlement of fucoid sporelings which, in the absence of many limpets, grew into a rich growth of fucoids. The sweeping action of the fucoids limited settlement of barnacles which inhibit the movement of limpets 65Pv , so P. vulgata on neighbouring rocks were able to easily move in at the margins to feed on fucoid sporelings and thalli. There was some reduction in fucoid cover in summer 1948. Gradually over succeeding years P. vulgata regained its dominance and the algal cover was reduced to its former level. In 1967, an oil tanker was wrecked in Cornwall. The resultant pollution and its treatment killed the limpets on affected shores, and a similar sequence of events to that in the Isle of Man was observed (Yonge & Thompson, 1976).
Experimental or accidental destruction aside, at mid tide level on moderately exposed rocky shores in north-west Europe there is a natural cycle over years of complex interactions between P. vulgata, Semibalanus balanoides and Fucus vesiculosus leading to patchy and fluctuating distributions (Hawkins et al., 2008) and impact on other organisms living with them. In south-west Britain and southern Europe, where P. depressa replaces P. vulgata as the dominant limpet, dense patches of fucoids are less likely to occur than on more northern shores (Jenkins et al. 2005).
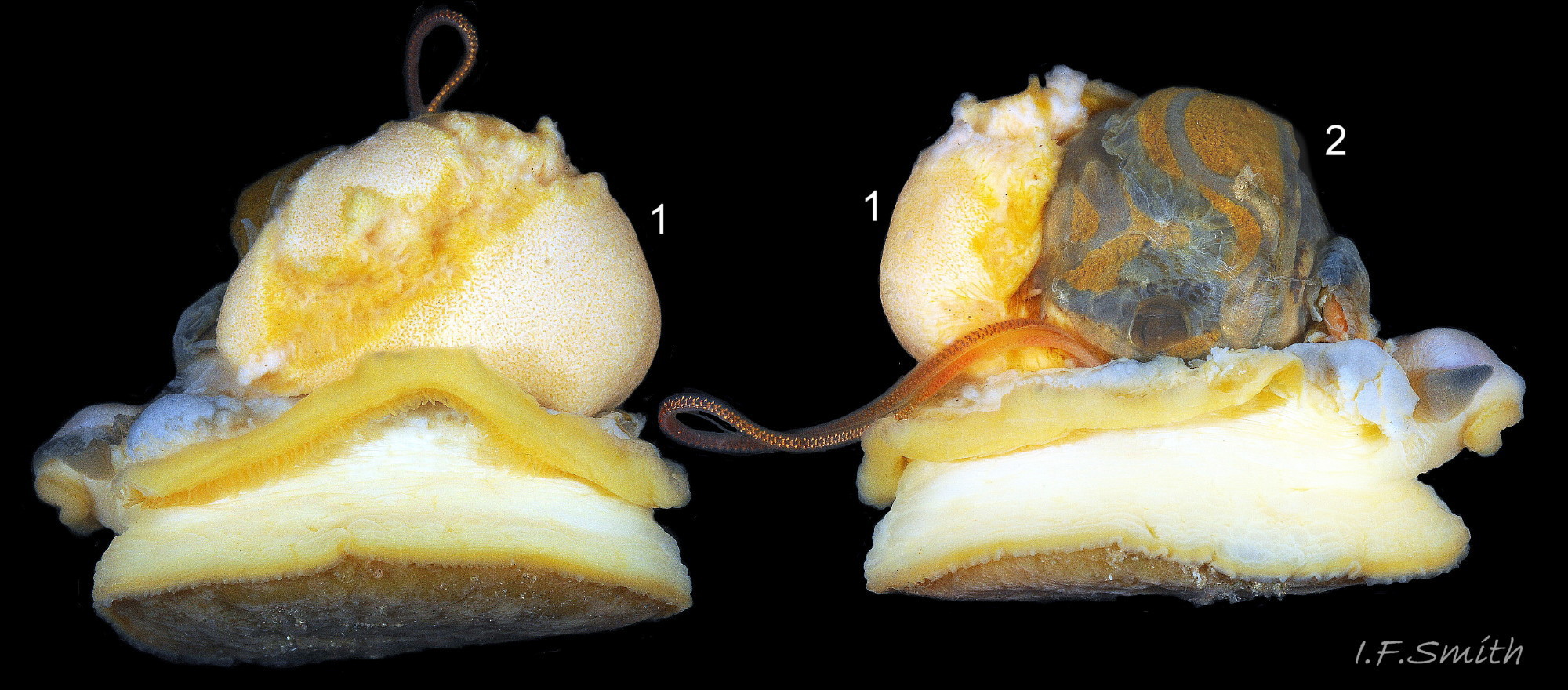
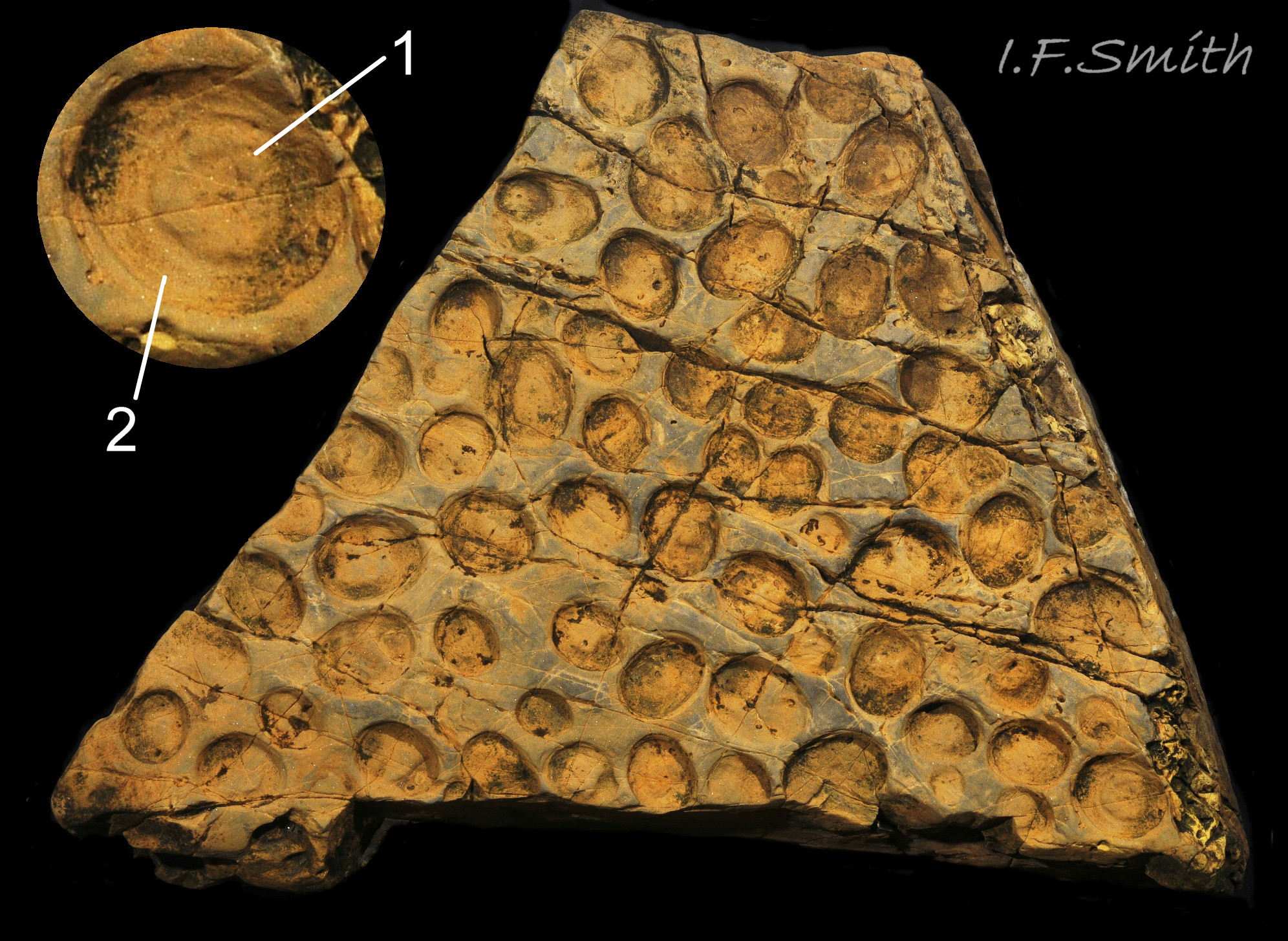
Predators able to dislodge limpet shells include gulls, wrasse, oystercatchers and rats. Nucella lapillus can force entrance for its radula under the shell rim of small limpets, but not those longer than about 35 mm (Flint, 2001) which they bore through with rasping of the radula and chemicals and enzymes from an accessory boring organ. In the great majority of cases, they bore the dorsal part of the shell through the pedal-retractor muscle (PRM) and the area within its confines where the viscera are located. Of 73 vacant bored shells of Patella spp. examined in Guernsey only 7% of the holes were into the viscera-free periphery distal of the muscle, though the periphery was about 50% of the area of the shells (Flint, 2001) 66Pv . Boring takes several days, but is rewarded with a large food supply, providing the Nucella is not dislodged before completion. Boring affects large Patella to the greatest degree on sheltered shores where, perhaps partly consequently, limpets and their grazing are often less frequent and fucoids more plentiful (Flint, 2001 & Hawkins et al. 2009).
Respiration: cilia on the gills create gentle local inhalent respiratory water currents all around the perimeter of the animal from the adjacent shell-rim onto the gills. The cilia also create an exhalent current below the gills to remove the water via the shell-rim 67Pv (Yonge & Thompson, 1976). A densely ciliated groove on the gill margin catches and removes large particles of detritus that would clog the gill 28Pv (Fretter & Graham, 1994).
Vascular system: colourless haemolymph depleted of oxygen leaves the foot-head through gaps between the bundles of the pedal retractor muscle and enters the afferent branchial vessel along the sides of the PR muscle 29Pv. Thence it passes through the pallial gills where it receives oxygen, which turns it bluish, before passing into the efferent branchial vessel which encircles the entire animal 30Pv . The haemolymph passes from the efferent vessel through a large trunk into the heart located in the nuchal cavity near the left anterior muscle bundle. The heart pumps the oxygenated haemolymph to the organs of the foot-head
Breeding varies geographically; winter in southern England, September in north-east England and August in northern Scotland with variation from year to year (Fretter & Graham, 1994). P. vulgata is a protandrous hermaphrodite; usually changing from male to female at ages 1 to 3 years, occasionally later or not at all (Fretter & Graham, 1976). Fertilization is external so there is no penis. Sperm and individual ova are shed into the water column. Eggs hatch after 24 hours into free trochophore larvae (the stage passed within the egg by most “less-primitive” spp.) in the plankton. At 15 days, still with a larval velum, they start crawling. At 3 weeks, metamorphosis is complete and the shell is 0.2 mm long. Growth varies with the availability of bare rock free of barnacles suitable for grazing. In a study at Bantry Bay, Ireland, shell lengths of those on bare rock were 11-18 mm at 1 year, 20-30 mm at 2 years and 24-34 mm at 3 years, but those restricted by barnacle cover at the same time intervals were only 3-6 mm, 7-12 mm and 11-15 mm (Thompson, 1980). Early stages are usually in damp situations or rock pools, some moving up the shore to drier situations later in life 61Pv .
Distribution and status
P. vulgata lives all round Britain and Ireland where there are suitable bedrock, boulders or stable shingle for attachment. In the north-east Irish Sea and between the Humber and Thames estuaries it is the only species of rock dwelling Patella; NBN UK map includes misidentified P. ulyssiponensis and P. depressa https://species.nbnatlas.org/species/NHMSYS0021056399 . In Britain and Ireland it is the dominant species except on some shores of south-west England where P. depressa is dominant and on some exposed shores where P. ulyssiponensis is the main species. Except those on weed drifted from afar, P. vulgata is probably the only rock dwelling species of Patella living from Lower Normandy to Denmark with few or none north of Rotterdam. It is absent from the Baltic, but occurs in Atlantic Scandinavia to the north of Norway. The southern limit of P. vulgata is the south coast of Portugal (Cabral & Simões, 2007, Lima et al., 2016 and Oróstica, 2020) where “the distribution of P. vulgata – – – appears to depend directly on the temperature in May, the species being absent when the temperature is higher than ca. 18°C” (Cabral & Simões, 2007). There are many misidentified records of it in southern Spain and the Mediterranean on GBIF https://www.gbif.org/species/5190391.
Appendix; comparison of authors’ descriptions.
Shell exterior
1) Barrett & Yonge(1958). Up to 65 mm long. Shell typically tall, though variable.
2) Eales(1967). Up to 65 mm long. A rather tall cone. Ribs from apex to margin, with smaller ones between the larger.
3) McMillan (1968). Usually up to 50 mm, exceptionally 70 mm.
4) Beedham (1972). Up to 55 mm long. Usually rather tall but varies. Strong ribs. Yellowish green but very variable.
5) Graham (1988). Up to 50 mm long. Shell high to low. Apex more or less central. Strong radial ridges. White or grey with dark paired bands in furrows.
6) Fish & Fish(1989). Up to 60 mm long. Apex central or slightly anterior. Radiating ridges. Whitish grey or fawn.
7) Hayward & Ryland (1995). Up to 60 mm long. Apex central or slightly anterior. Irregular radial ribs.
8) Trigo et al. (2018). Up to 60 mm long. Shell usually very raised. Irregular radial ribs.
9) Wiese & Janke (2021). Up to 40 mm long, occasionally over 60 mm. Height-width ratio varies greatly, apex angle 70 – 115°. Numerous radial ribs. Yellowish-brown with darker radii. Often encrusted.
Shell interior
1) Barrett & Yonge (1958). White or yellowish. Head scar white to brown.
2) Eales(1967).Yellow or white. Central body scar white to dark brown.
3) McMillan(1968). Varies much but never white or golden yellow. Head scar never orange. [Colour plate 1 fig. 1 is P. vulgata with silver grey vertex patch mislabelled as P. aspera =P. ulyssiponensis]
4) Beedham(1972). White or yellowed. Vertex patch silvery nacreous or opaque white.
5) Graham(1988). Aperture oval, narrower anteriorly, smoothly rounded behind. Dull greenish brown, grey-green or yellowish, white towards the centre. External dark bands may be visible internally.
6) Fish & Fish (1989). Greenish blue nacre, may be yellowish in older shells. Silvery apical area.
7) Hayward & Ryland (1995). Grey-green with bluish iridescence.
8) Trigo et al. (2018). Yellowish. Sometimes with dark radiating bands.
9) Wiese & Janke (2021). Shiny yellow-grey-green, sometimes a little iridescent.
Body
1) Barrett & Yonge (1958). Pallial tentacles transparent.
2) Eales(1967). Body brownish yellow.
3) McMillan (1968). Animal not black or dark-coloured.
4) Beedham (1972). Transparent pallial tentacles, lacking white pigment. Foot light greyish green.
5) Graham(1988). Pallial tentacles devoid of white pigment. Foot dusky.
6) Fish & Fish (1989). Pallial tentacles transparent. Foot grey, greyish green or yellowish.
7) Hayward & Ryland (1995). Translucent or grey pallial tentacles. Foot olive, grey or khaki.
8) Trigo et al. (2018). Usually lighter than P. depressa.
9) Wiese & Janke (2021).
Only four of the above sources have coloured photographs of P. vulgata. They are more useful than words for depicting colours, but available space prevented display of the wide variation which occurs in P. vulgata.
3) McMillan(1968). Single top view of shell exterior and views of two shell interiors (one misidentified).
6) Fish & Fish (1989), Single image of foot and pallial tentacles with comparative images of P. ulyssiponensis and P. depressa.
8) Trigo et al. (2018). Exterior top and lateral, and interior views of a single specimen.
9) Wiese & Janke (2021). Exterior top and lateral, and interior views of a single specimen.
Acknowledgements
I thank David Fenwick https://www.aphotomarine.com/ , Judy Fenerty, Joel Roane and Libby Scarfe for use of images, and I thank Stephen J. Hawkins, Alan Hodgson and André Ampuero León for helpful information.
Links and references
Davis, J.R.A. and Fleure, H.J. 1903. Patella. LMBC Memoir 10. Liverpool University Press. https://archive.org/details/lmbcmemoirsontyp10live
Ballantine, W.J. 1961. A biologically-defined exposure scale for comparative description of rocky shores. Field studies 1(3): 1-19.
https://cdn.fieldstudiescouncil.net/fsj/vol1.3_17.pdf
Cabral, J.P. and Simões, J. 2007. The southern limit of distribution of Patella vulgata. Iberus, 25 (1): 57-75.
Barrett, J. and Yonge, C.M. 1958 Collins pocket guide to the sea shore. London, Collins.
Beedham, G.E. 1972. Identification of the British Mollusca. Amersham, Hulton Educational Publications.
Branch, G.M. 1981a. The biology of limpets. Oceangr. Mar. Biol. Ann. Rev. <b19: 235-380. Part 1
Branch, G.M. 1981b. The biology of limpets. Oceangr. Mar. Biol. Ann. Rev. <b19: 235-380. Part 2 https://www.google.co.uk/search?q=Patella+vulgata+blood+circulation&ie=utf-8&oe=utf-8&gws_rd=cr&ei=QoPIVe6GBMP7UNGcrKAF
Flint, D. 2001. Unpublished study of Nucella predation on Patella spp. in Guernsey.
Forbes, E. & Hanley S. 1849. A history of the British mollusca and their shells. vol. 2, London, van Voorst. 421-425 https://archive.org/details/historyofbritish02forb/page/421/mode/1up?view=theater
Fretter, V. and Graham, A. 1962. British prosobranch molluscs: their functional anatomy and ecology. London, Ray Society. [Includes species index.]
Fretter, V. and Graham, A. 1976. The prosobranch molluscs of Britain and Denmark. Part 1 – Pleurotomariacea, Fissurellacea and Pattellacea. Suppl. 1, J. Moll. Stud..
Fretter, V. and Graham, A. 1994. British prosobranch molluscs. London, Ray Society. [Revised and updated edition, but no species index.]
Goshima, S., Ilano A.S. & Ito, A. 2002. Seasonaland tidal-height variationsin body weight and radular lengthin Nodilittorina radiata (Eydoux & Souleyet, 1852) J. Mollus. Stud. 68(3): 197-203.
https://academic.oup.com/mollus/article/68/3/197/1004439?login=false
Graham, A. 1988. Prosobranch and pyramidellid gastropods. London.
Haszprunar, G. 1985. The Fine Morphology of the Osphradial Sense Organs of the Mollusca. I. Gastropoda, Prosobranchia. Phil. trans. R. Soc. Lond. B. 307 457-496. https://www.researchgate.net/publication/247605282_The_Fine_Morphology_of_the_Osphradial_Sense_Organs_of_the_Mollusca_I_Gastropoda_Prosobranchia
Hawkins, S.J., Moore, P.J., Burrows M. T., Poloczanska E., Mieszkowska N.,
Herbert R.J.H., Jenkins S.R., Thompson R.C., Genner M.J. and Southward A.J. 2008. Complex interactions in a rapidly changing world: responses of rocky shore communities to recent climate change. Climate Research 37:123–133. https://www.int-res.com/articles/cr_oa/c037p123.pdf
Hawkins, S.J.,Sugden, H.E., Mieszkowska, N., Moore, P.J., Poloczanska, E., Leaper, R., Herbert, R.J.H., Genner, M.J., Moschella, P.S., Thompson, R.C., Jenkins, S. R., Southward, A. J. and Burrows, M. T. 2009. Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Mar Ecol Prog Ser 396: 245–259. https://www.researchgate.net/publication/216619219_Consequences_of_climate_driven_biodiversity_changes_for_ecosystem_functioning_of_North_European_Rocky_Shores
Hawkins, S.J. (narrator) 2013. The secret life of rock pools BBC4; YouTube video https://youtu.be/vd7KkAKSIiA?si=rkvkuuq7vR8DVeuY [Copy and paste link into a browser]
Jeffreys, J.G. 1865. British conchology. vol. 3, 236-241 . London, van Voorst. https://archive.org/details/britishconcholog03jeff/page/236/mode/1up?view=theater
Jenkins, S.J., Coleman, R.A., Della Santina, P., Hawkins, S.J., Burrows, M.T. and Hartnoll, R.G. 2005. Regional scale differences in the determinism of grazing effects in the rocky intertidal. Mar Ecol Prog Ser 287: 77–86
Jones, N. S. 1948. Observations and experiments on the biology of Patella vulgata at Port St Mary, Isle of Man. Proceedings and Transactions of Liverpool Biological Society, 56, 60–77.
Kendall, M.A., Burrows, M.T., Southward, A.J., & Hawkins, S.J. 2004. Predicting the effects of marine climate change on the invertebrate prey of the birds of rocky shores. Ibis 146 (Suppl.1): 40 – 47.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1474-919X.2004.00326.x
Lewis, J.R. 1964. The ecology of rocky shores. London, Hodder & Stoughton.
Light, J. A guide to limpet identification for the general naturalist http://www.glaucus.org.uk/Limpet.htm
Lima, F.P., Gomes, F., Seabra, R., Wethey D.S., Seabra, M.I., Cruz, T., Santos, A.M. and Hilbish, T.J. 2016. Loss of thermal refugia near equatorial range limits. Global Change Biology 22: 254–263.
MacClintock, C. 1967. Shell structure of patelloid and bellerophontid gastropods (Mollusca). Peabody Museum of Natural History, Yale University. Bulletin 22. https://elischolar.library.yale.edu/peabody_museum_natural_history_bulletin/22/
McKay, D.W. and Smith, S.M. 1979. Marine mollusca of East Scotland. Edinburgh, Royal Scottish Museum.
Oróstica, M. H 2018. Living at the edge: Ecology of Patella species in Britain. PhD thesis. Bangor University. https://research.bangor.ac.uk/portal/files/22310847/2018OrosticaPhD_1.pdf
Oróstica, M. H., Hawkins, S. J., Broitman, B. R. and Jenkins S. R. 2020. Performance of a warm-water limpet species towards its poleward range edge compared to a colder-water congener. Mar Ecol Prog Ser https://doi.org/10.3354/meps13461
Sanna, D., Dedola, G. L., Lai, T., Curini-Galletti, M. & Casu, M. 2011. PCR-RFLP: A practical method for the identification of specimens of Patella ulyssiponensis s.l. (Gastropoda: Patellidae), Italian Journal of Zoology,
https://www.researchgate.net/publication/233126771_PCR-RFLP_A_practical_method_for_the_identification_of_specimens_of_Patella_ulyssiponensis_sl_Gastropoda_Patellidae
Sá-Pinto, A., Branco,M., Harris, D.J. & Alexandrino, P. 2005. Phylogeny and phylogeography of the genus Patella based on mitochondrial DNA sequence data. J. Exp. Mar. Biol. Ecol. 325: 95-110.
Sá-Pinto, A., Alexandrino, P. & Branco,M. 2007. High genetic differentiation with no evidence of hybridization between four limpet species (Patella spp.) revealed by allozyme loci. Scientia Marina 71(4): 801-810. Barcelona. www.vliz.be/imisdocs/publications/131981.pdf
Smith, I.F. 2015. Limpet shell structure. https://flic.kr/s/aHskjiP2GM
Thompson, G.B. 1980. Distribution and population dynamics of the limpet Patella vulgata in Bantry Bay. J. exp. mar. Biol. Ecol., 45 173-217
https://www.sciencedirect.com/science/article/abs/pii/0022098180900581
Trigo, J.E.; Diaz Agras, G.J.; Garcia Alvarez, O.L.; Guerra, A.; Moreira, J.; Pérez, J.; Rolán, E.; Troncoso, J.S,; Urgorri, V.. 2018. Guia de los Moluscos Marinos de Galicia. Servicio de Publicacións da Universidade de Vigo.
Wiese, V. and Janke, K. 2021. Die Meeresschnecken und –muscheln Deutschlands Wiebelsheim, Quelle & Meyer.
Yonge, C.M. and Thompson, T.E. 1976. Living marine molluscs. London.
GLOSSARY
afferent = conducting inwards or towards an organ.
amphora = ancient Greek jar with large oval body and thin neck
aperture = mouth of gastropod shell; outlet for head and foot.
apex = earliest formed part of shell, summit of exterior of Patella cone.
cartilages = structures of tough, resilient material, histologically resembling vertebrate cartilage, form bolsters of the odontophore.
cartilaginous = made of, or resembling, cartilage.
cephalic = (adj.) of or on the head.
cilia = (pl.) microscopic linear extensions of membrane which move in waves to create locomotion, or move particles and liquids (SEM image at https://flic.kr/p/qQB5zj )
ciliary = (adj.) relating to or involving cilia.
conoid = shaped like a cone.
ctenidium = comb-like molluscan gill. Usually an axis with a row of filaments either side, not found on Patella species.
cuticle = non-cellular waxy layer secreted by epidermal cells.
cuticularized = altered into cuticle.
efferent = conducting outwards or away from an organ
ELWS = extreme low water spring tide level, usually near equinoxes.
EHWS = extreme high water spring tide level (usually near March and September equinoxes).
emersed = not covered by water.
epidermal = of or relating to the epidermis.
epidermis = thin outer layer of the skin.
epipodial = (adj.) of the epipodium (collar or circlet running round sides of foot of some gastropods).
epithelium = tissue forming outer layer of the body surface, organs and blood vessels, and inner surfaces of alimentary canal and other hollow structures.
haemolymph = circulating fluid in molluscs which carries nutrients, waste and hormones. Analogous to vertebrate blood, but most molluscs have copper-based haemocyanin in it, instead of red haemoglobin, to carry oxygen. It may be tinged blue when oxygenated; colourless when depleted of oxygen.
head scar = term used by many British authors for patch of different shell-material, and often different colour, near vertex of interior of limpet shell. Misnomer as the mobile head, free of any attachment to the shell or mantle-roof of the nuchal cavity cannot make a scar and is not located below the vertex. A preferable term is “vertex patch”.
height = (of limpet) perpendicular distance from apex to plane of aperture-rim; best measured with callipers or untilted lateral photograph.
hyaline shield = transparent sheet of chitin at anterior of radula that rests on bolsters of odontophore; attachment point for retractor muscles of radula.
immersed = covered by water.
jaw = unarticulated chitinous structure which encloses inner lips of Patella spp. at sides and anterior. Helps retain food particles cut by radula.
legit = (abbreviation; leg.) collected/ found by.
licker = cuticularized structure with plate-like ridges and deep transverse grooves at tip of radula of Patella spp.; retains and sweeps up food particles.
mantle = sheet of tissue covering visceral mass of molluscs. Secretes shell of shelled species.
mantle skirt = extension of mantle beyond viscera as a flap roofing nuchal cavity containing head, anus and genital and renal openings, and forming a groove for gills around the periphery of patellid limpets.
myostracum = scar made by the horseshoe-shaped pedal retractor muscle.
nuchal = (adj.) of nape of the neck.
nuchal cavity = cavity roofed by mantle skirt which contains head of limpet.
pedal retractor muscle = (abb. PRM, a.k.a. shell muscle, or columellar muscle) bundles of muscle arranged in a horseshoe which connect the shell to the foot.
pericardium = sac containing the heart.
porcelaneous = resembling vitreous glazed ceramic material.
protandrous = of a species in which individuals are initially male and become female later in life.
scar = mark on shell made by attachment point of muscle or mantle.
thallus = (pl. thalli) plant-like body of mature algae, which lacks plant features such as distinctly functioning stems, leaves and roots.
trochophore = spherical or pear-shaped larva that swims with aid of girdle of cilia. Stage preceding veliger, passed within gastropod egg in most species but free in plankton for patellid limpets.
tricuspid = (of teeth) having three points.
unicuspid = (of tooth) having a single point.
veliger = shelled larva of marine gastropod or bivalve mollusc which swims by beating cilia of a velum (bilobed flap).
vertex = angle at highest point on interior of limpet-shell; used to distinguish from the highest point, apex, on the exterior. Gmelin used “vertex” when describing the interior of P. ulyssiponensis, and in classical Latin “vertex” was used for the “pole of the heavens”; obviously only seen from below.
vertex patch = layer of different shell-material, and often different colour, at vertex of interior of limpet shell. (See “head scar”.)