Click image to enlarge with full caption. Main text below slider.
Testudinalia testudinalis (O.F. Müller , 1776).
Revised and Appendix added October 2020
Authors; Ian F. Smith (text) & Simon Taylor (shorework).
Current taxonomy: World Register of Marine Species (WoRMS)
www.marinespecies.org/aphia.php?p=taxdetails&id=234208
Synonyms: Patella testudinalis O.F Müller, 1776; Patella tessulata O.F Müller, 1776; Acmaea tessulata (O.F Müller, 1776); Acmaea testudinalis (O.F Müller, 1776); Collisella tessulata (O.F Müller, 1776); Lottia testudinalis (O.F Müller, 1776); Tectura tessulata (O.F Müller, 1776); Tectura testudinalis (O.F Müller, 1776); Testudinalia tessulata (O.F Müller, 1776);
Vernacular names: Northern tortoiseshell limpet (English); Brenigen fraith (Welsh); Schildkrötenschnecke (German); Skilpaddesnegl (Norwegian); Sköldpaddskålsnäcka (Swedish); Atlantic plate limpet (USA);
The former English vernacular was ‘Common tortoiseshell limpet’, but it is rare or absent in England so it was changed in October 2020 to ‘Northern’ on UK Species Inventory to reduce frequency of misidentification of Tectura virginea, the ‘White tortoiseshell limpet’ as Testudinalia testudinalis.
GLOSSARY below.
Shell Description
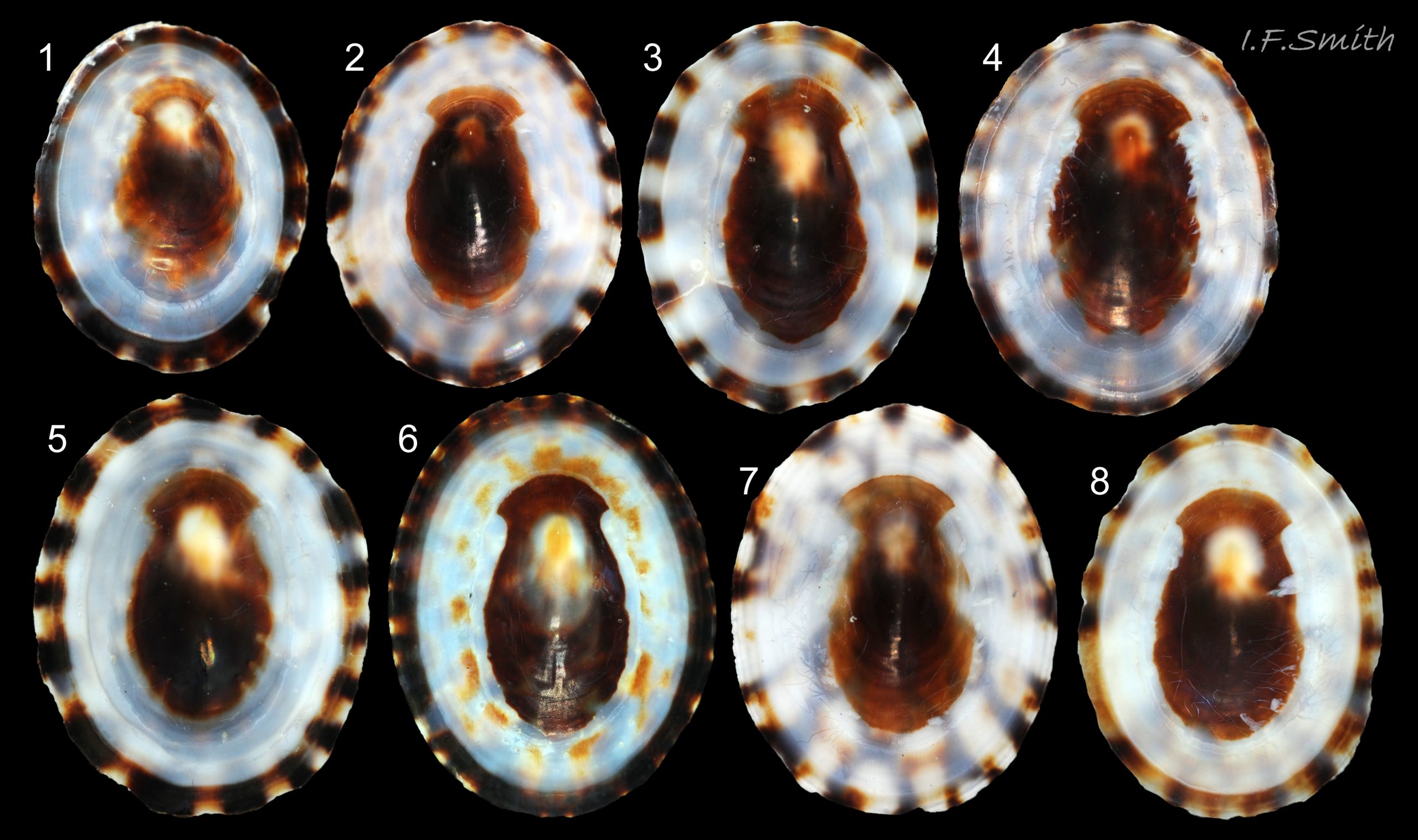
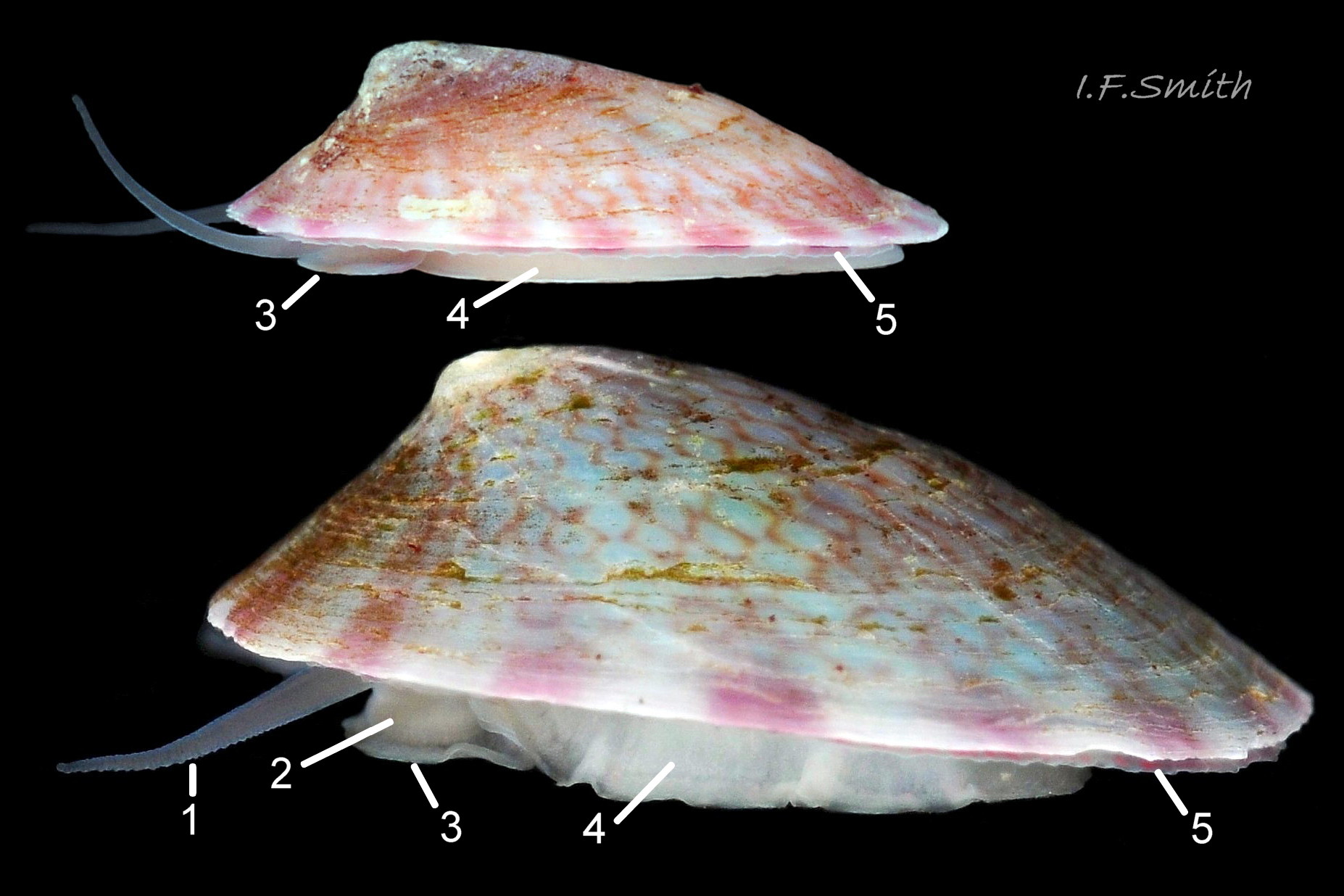
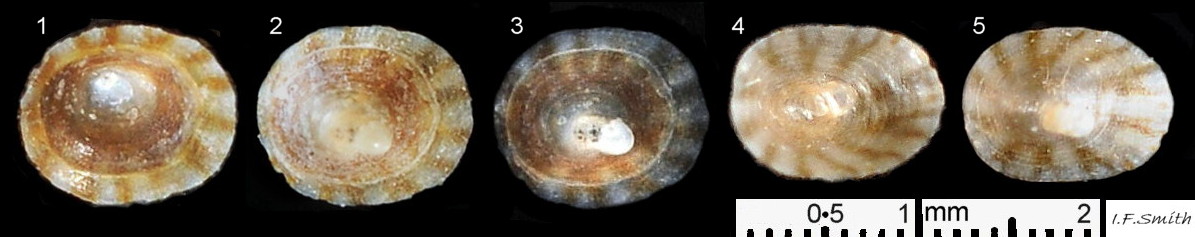
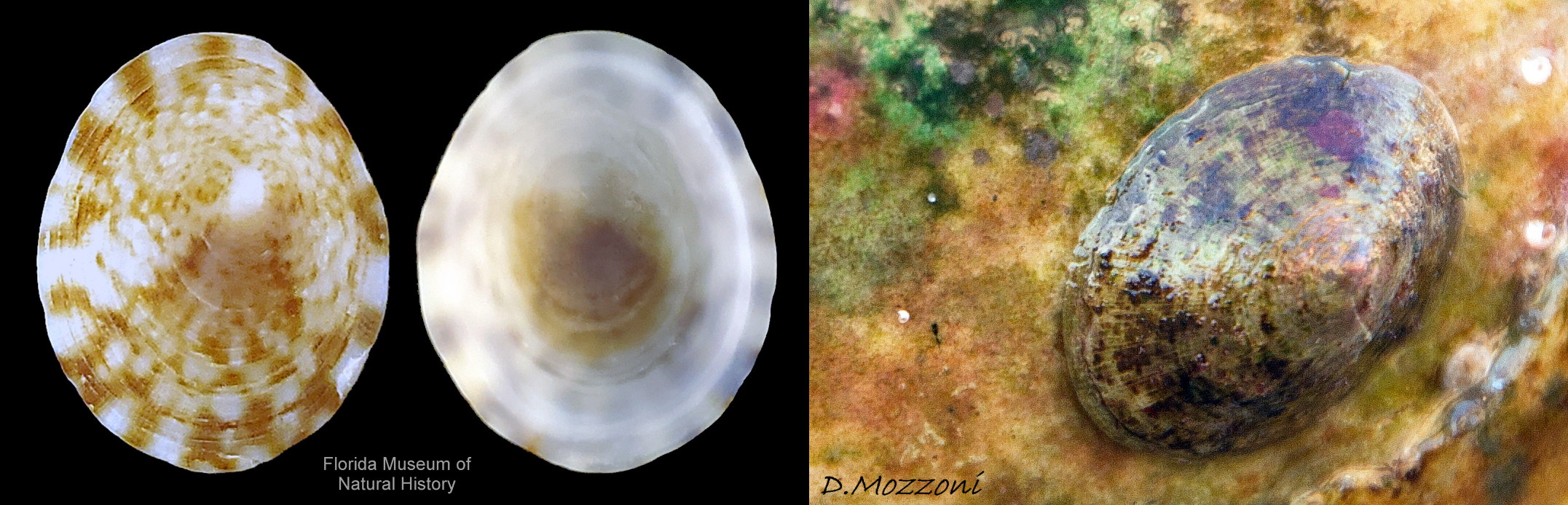
In Britain, sublittoral specimens usually up to 20mm long, 14mm wide, 10mm high, but 15mm is usual maximum length of intertidal specimens 01 Testudinalia testudinalis & 02 Testudinalia testudinalis. Extreme maximum length 30mm in Britain; may be larger in USA (Jeffreys, 1865). Shell rather thin and easily damaged when prising a specimen off substrate. Usually a low conoid, shell height about 25% to 36% of length 03 Testudinalia testudinalis. Eccentric apex tilted forwards, varies about 25% to 40% of shell-length from anterior. Minute spiral coil shell of veliger larva survives on apex until shell 1mm long. Aperture rim an ellipse; posterior and anterior usually similar breadth 04 Testudinalia testudinalis.
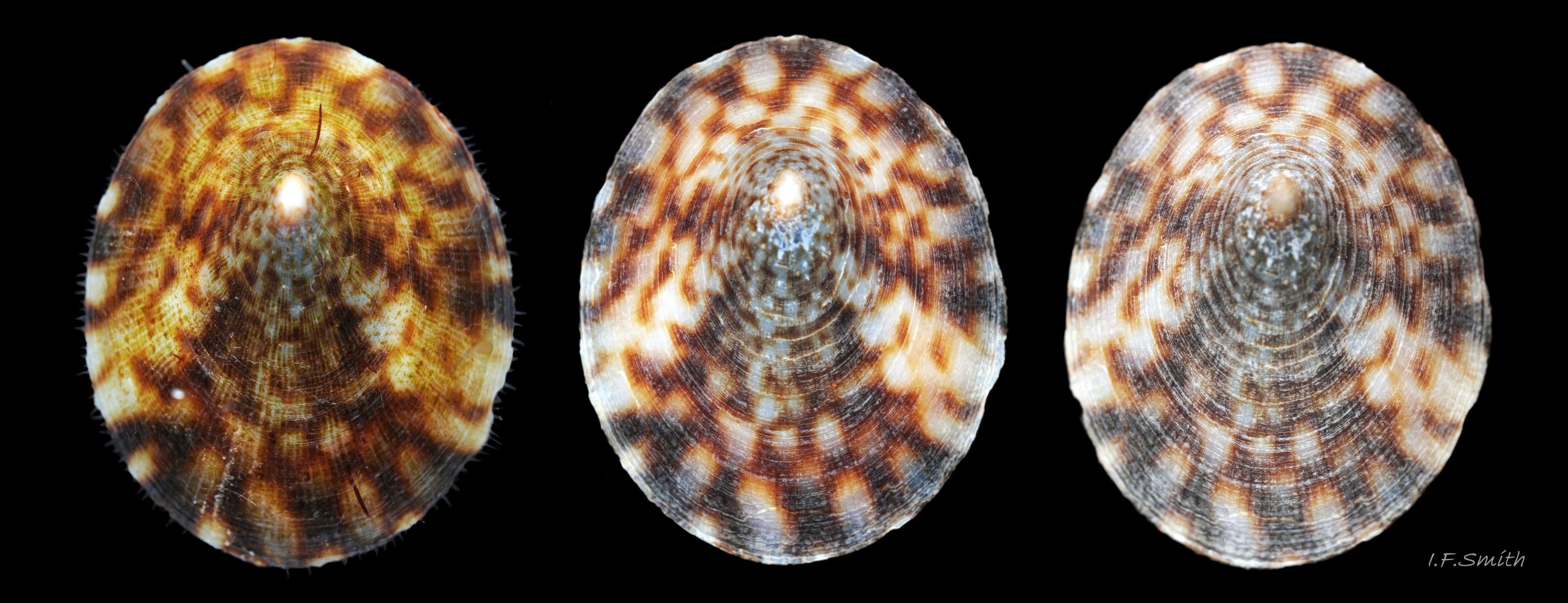
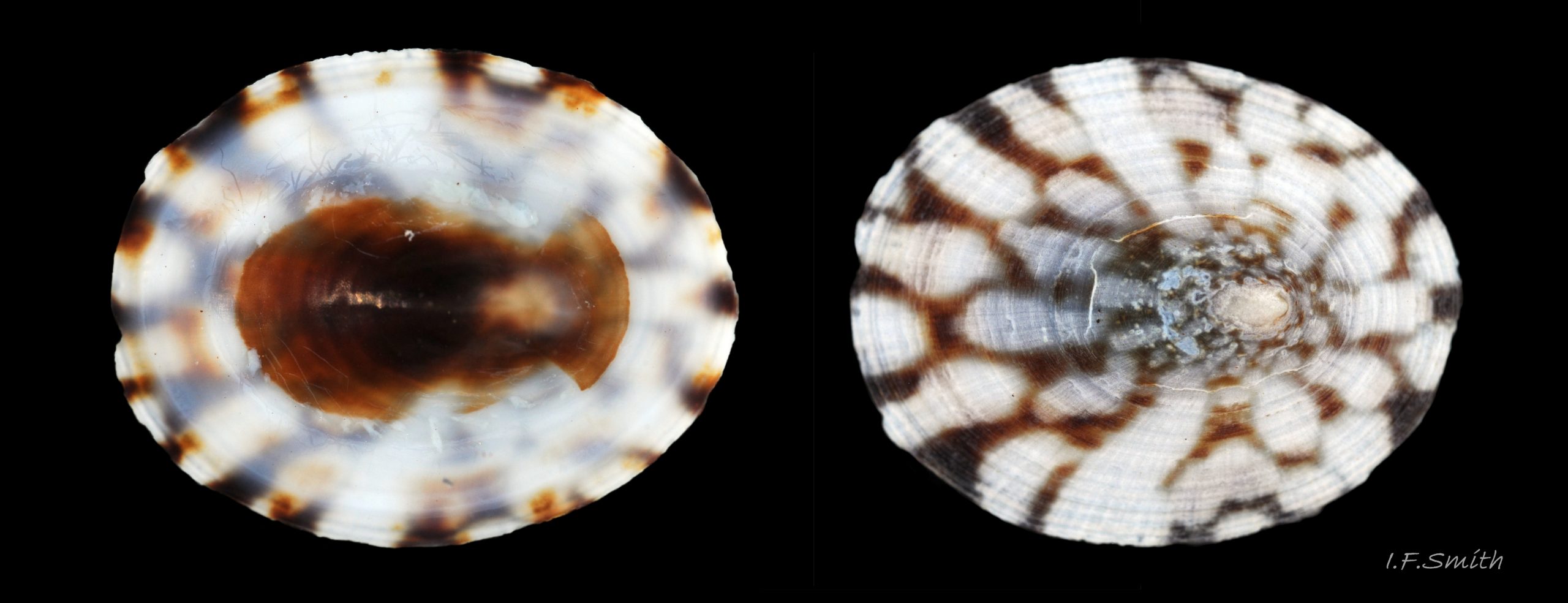
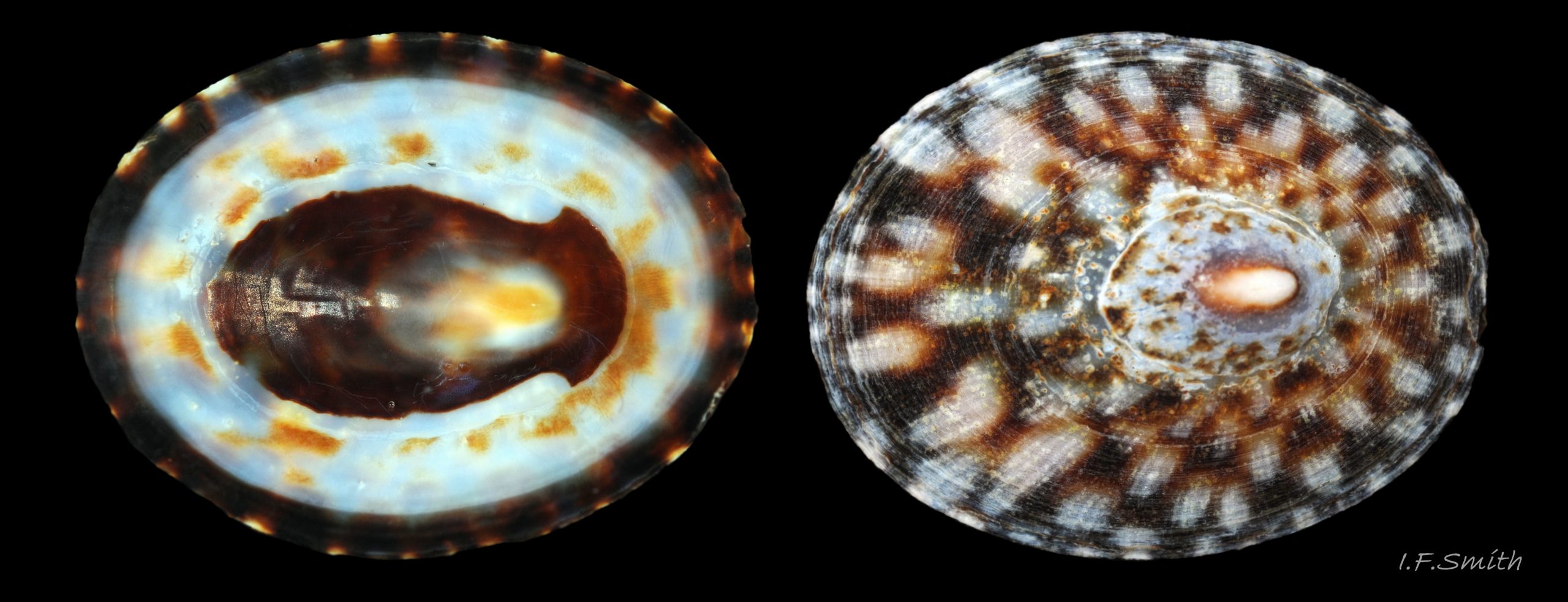
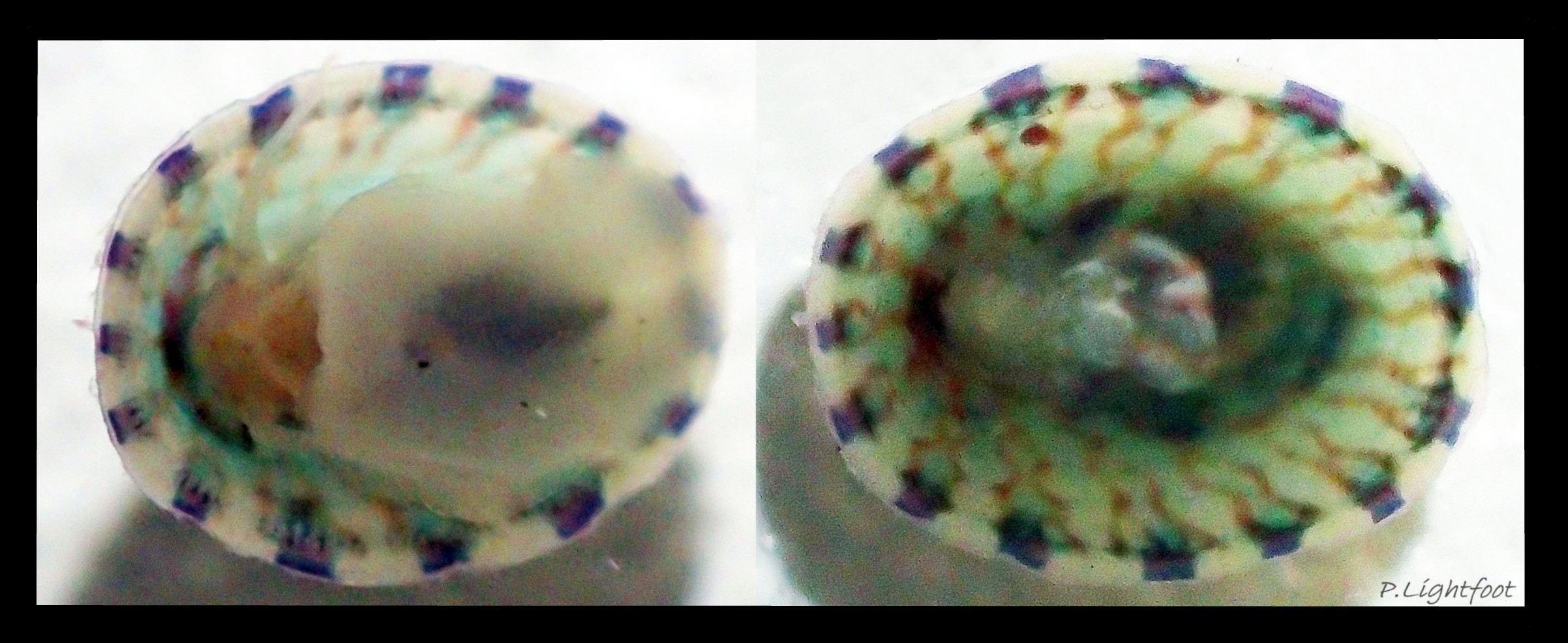
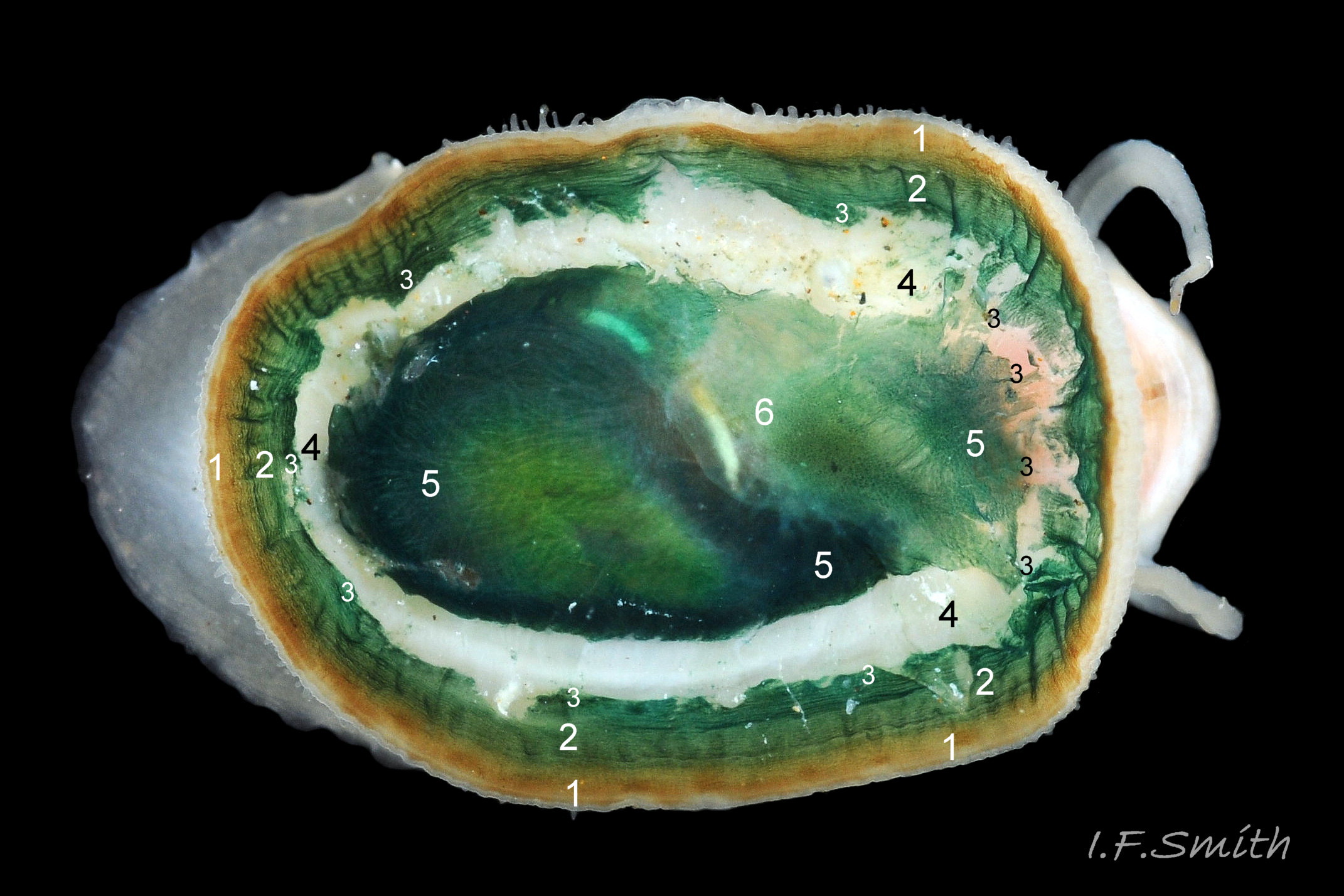
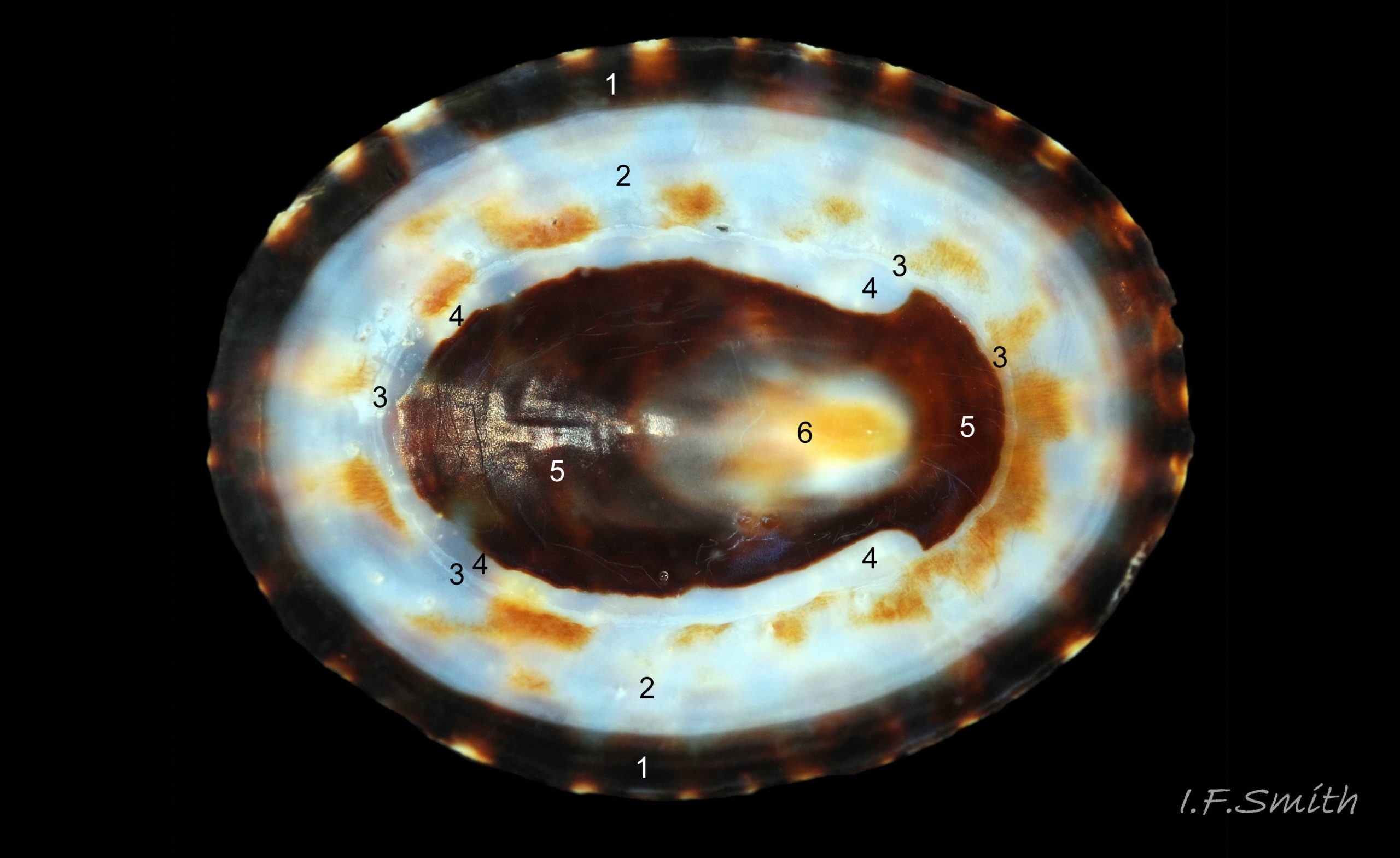
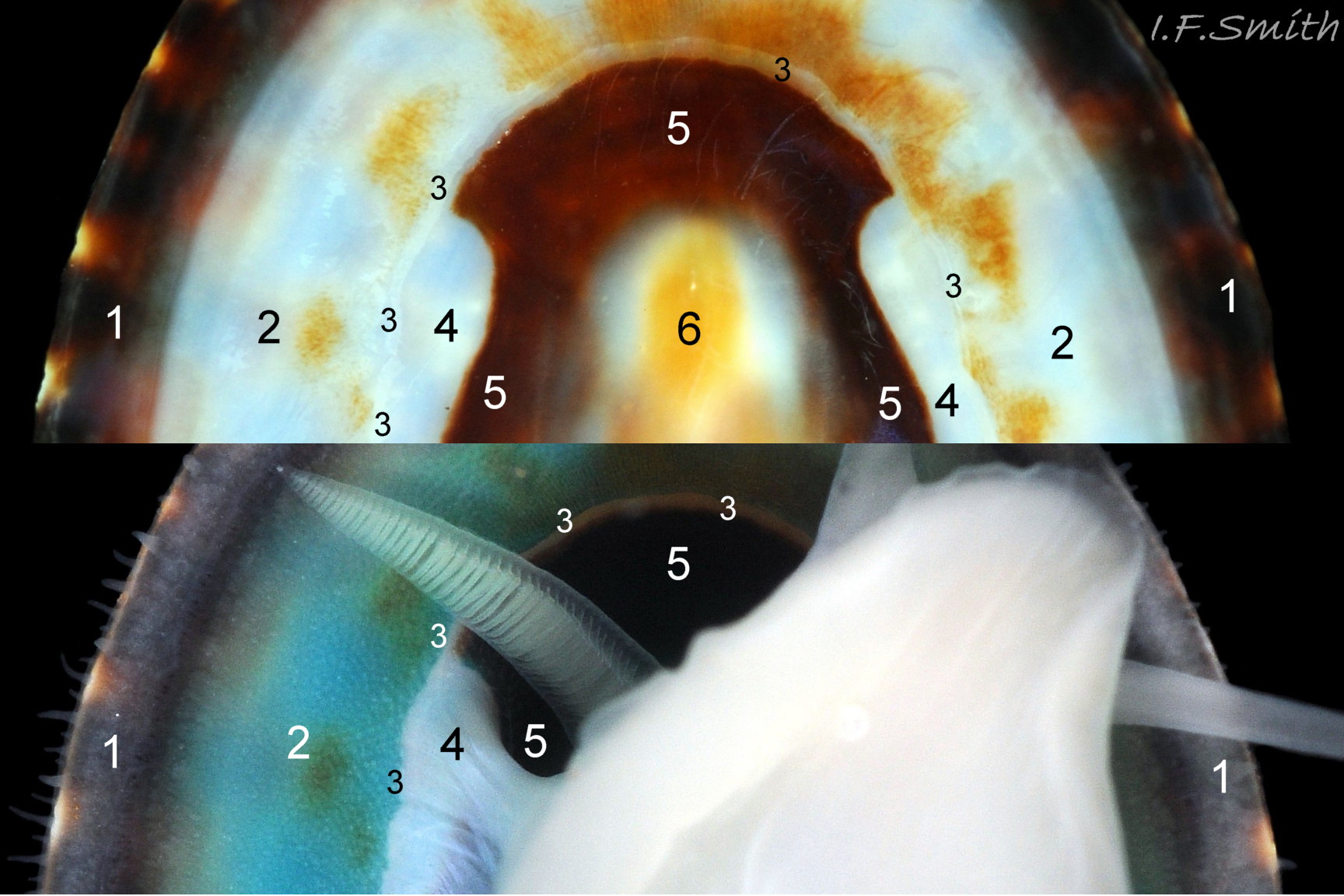
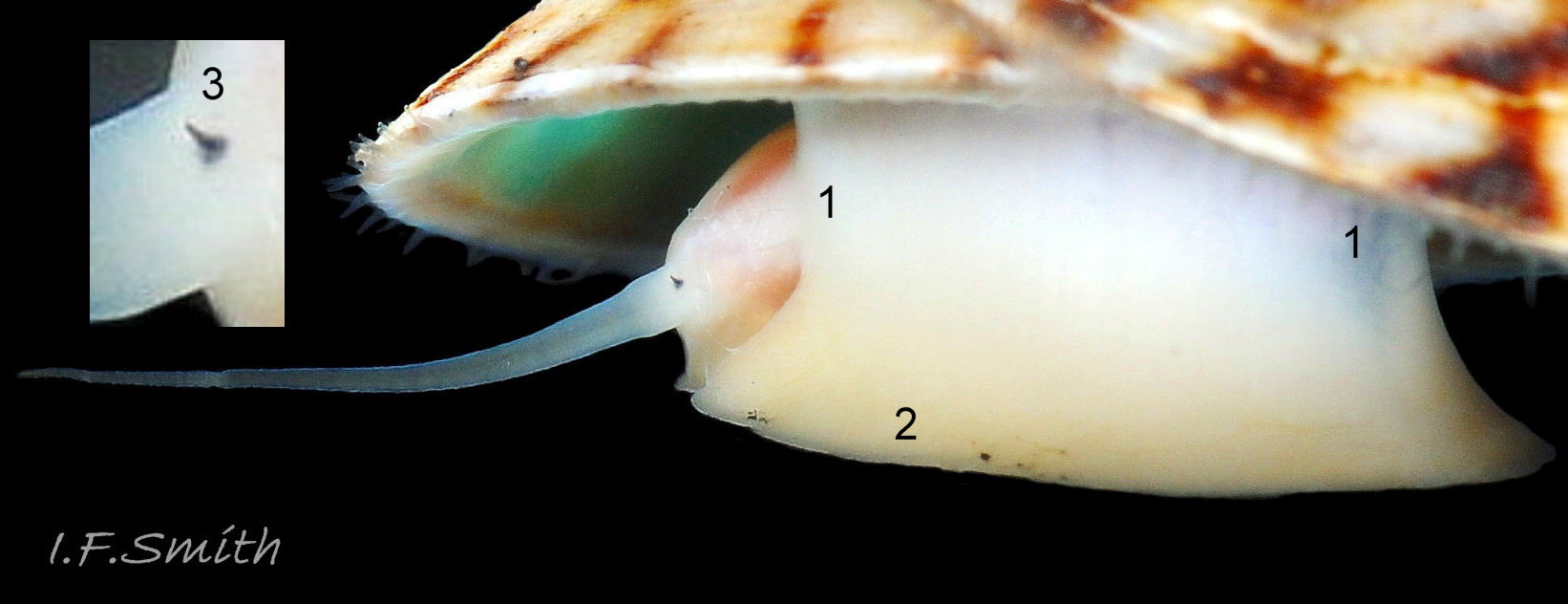
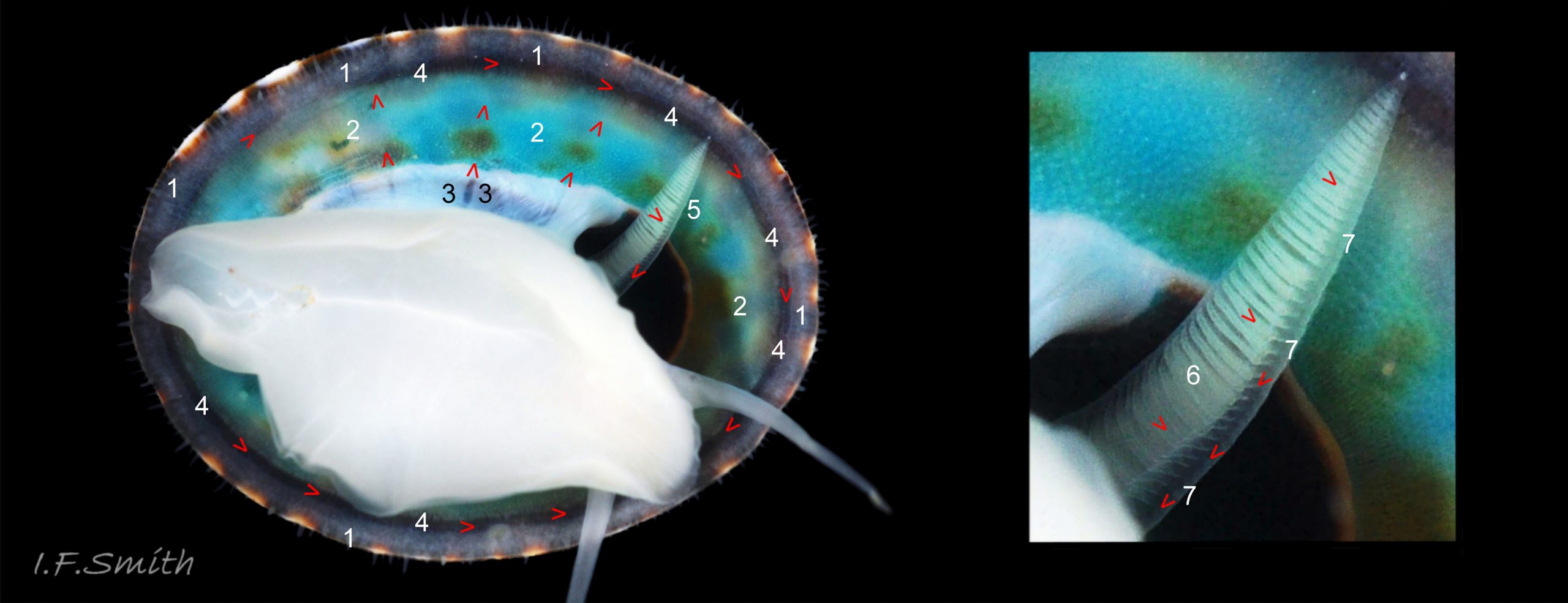
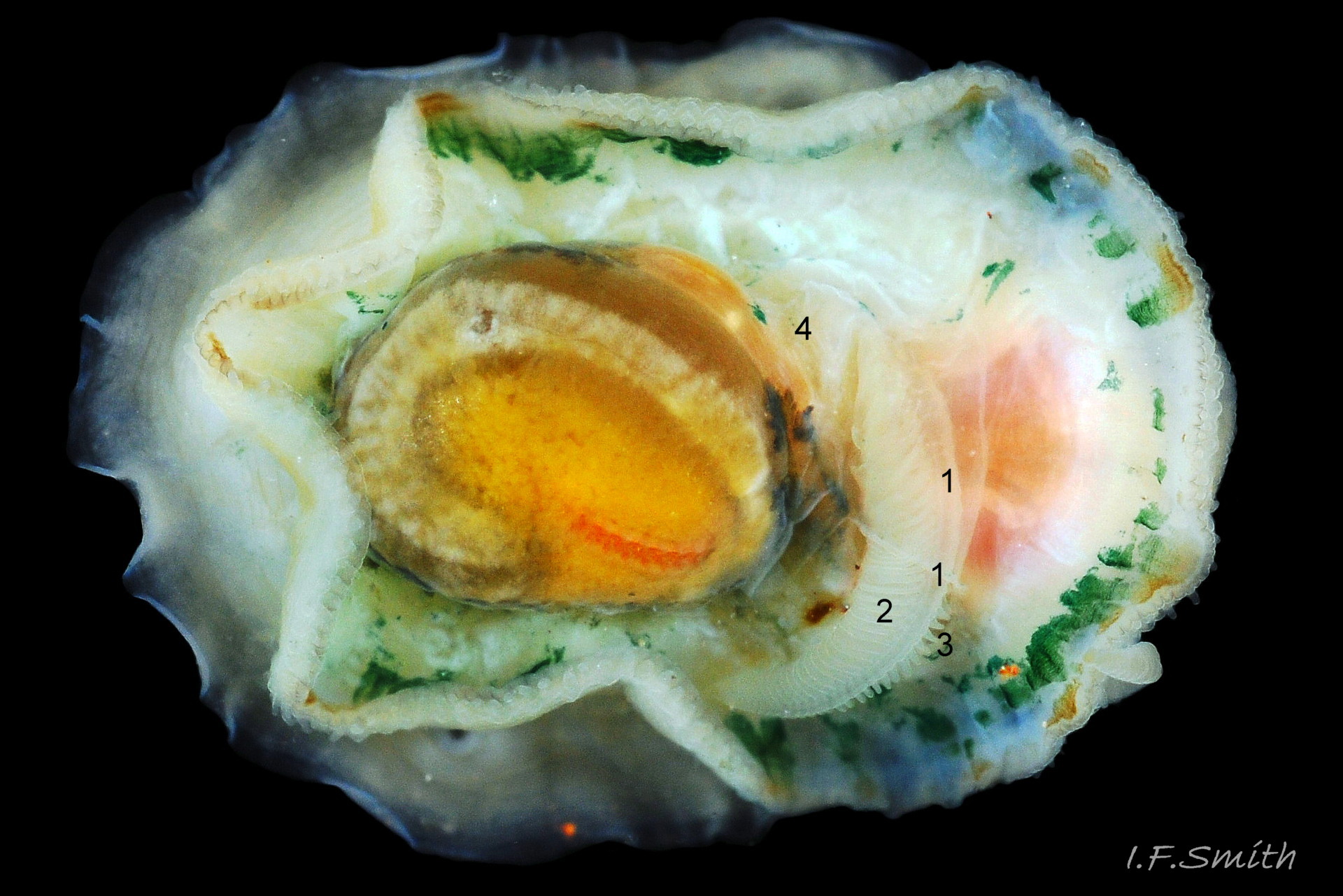
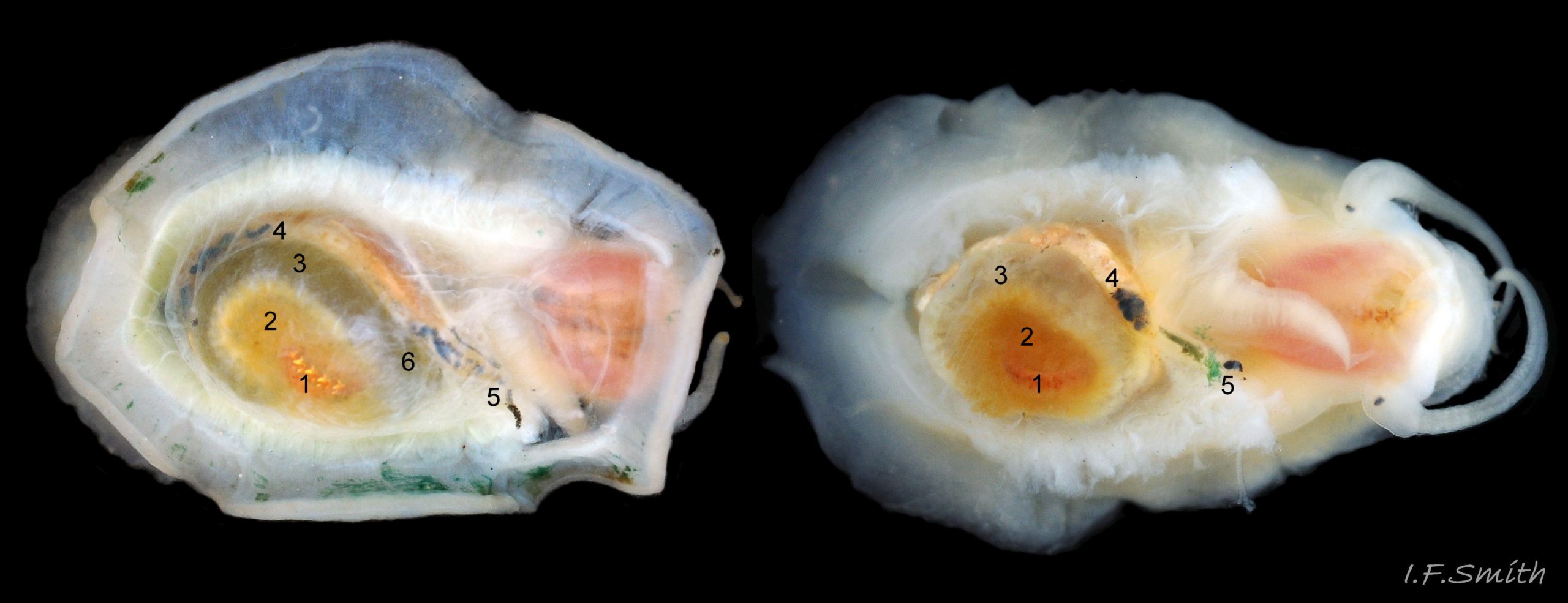
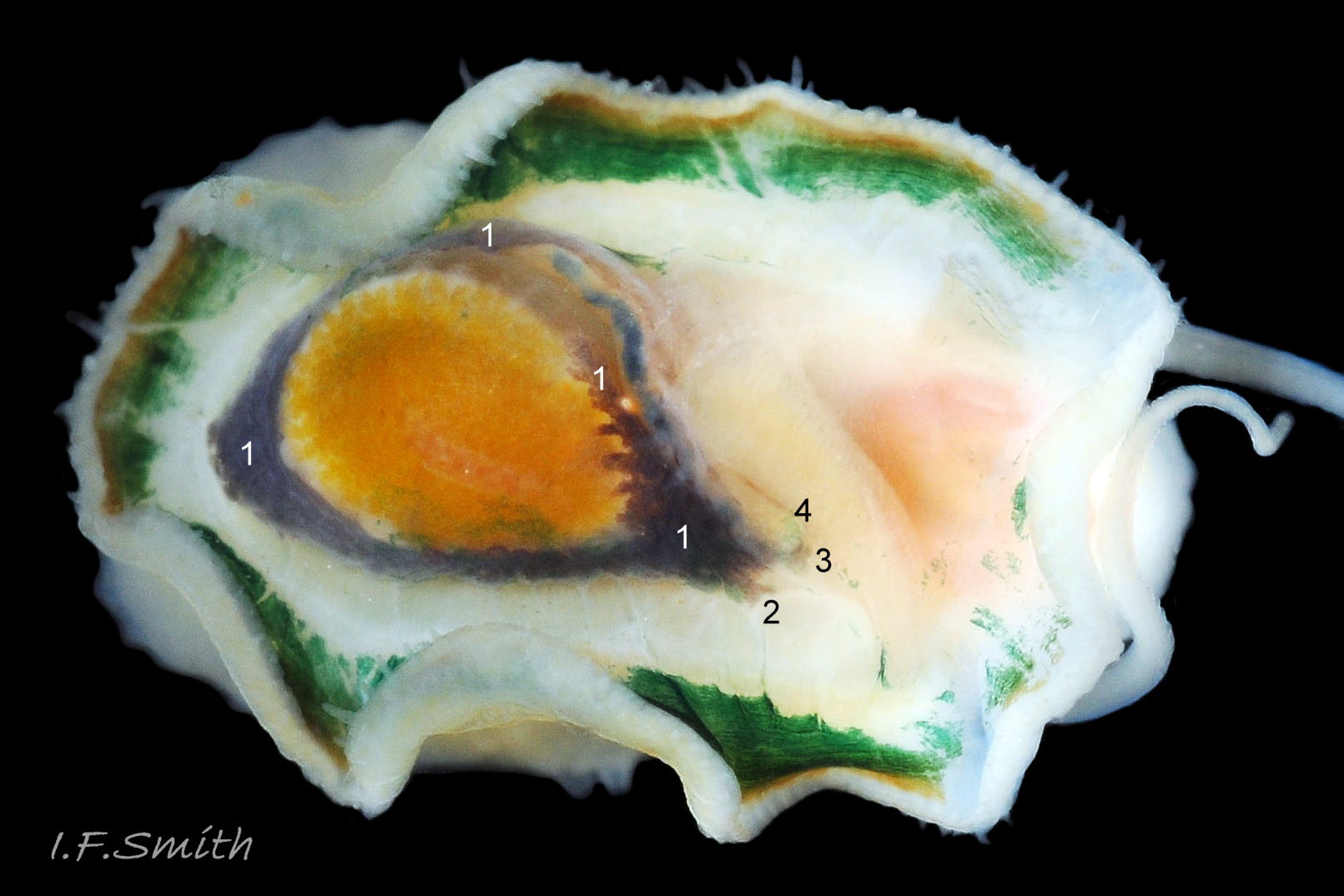
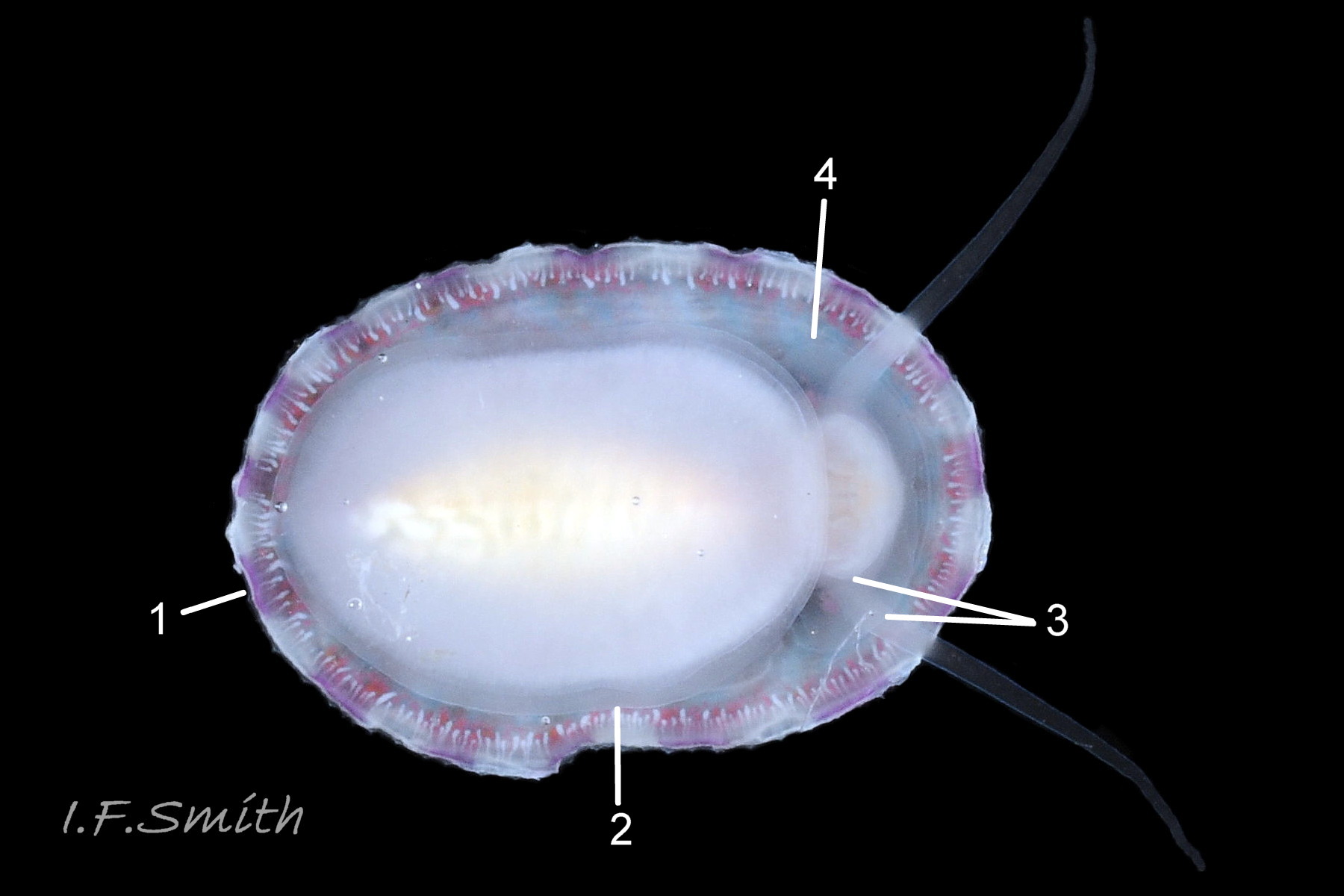
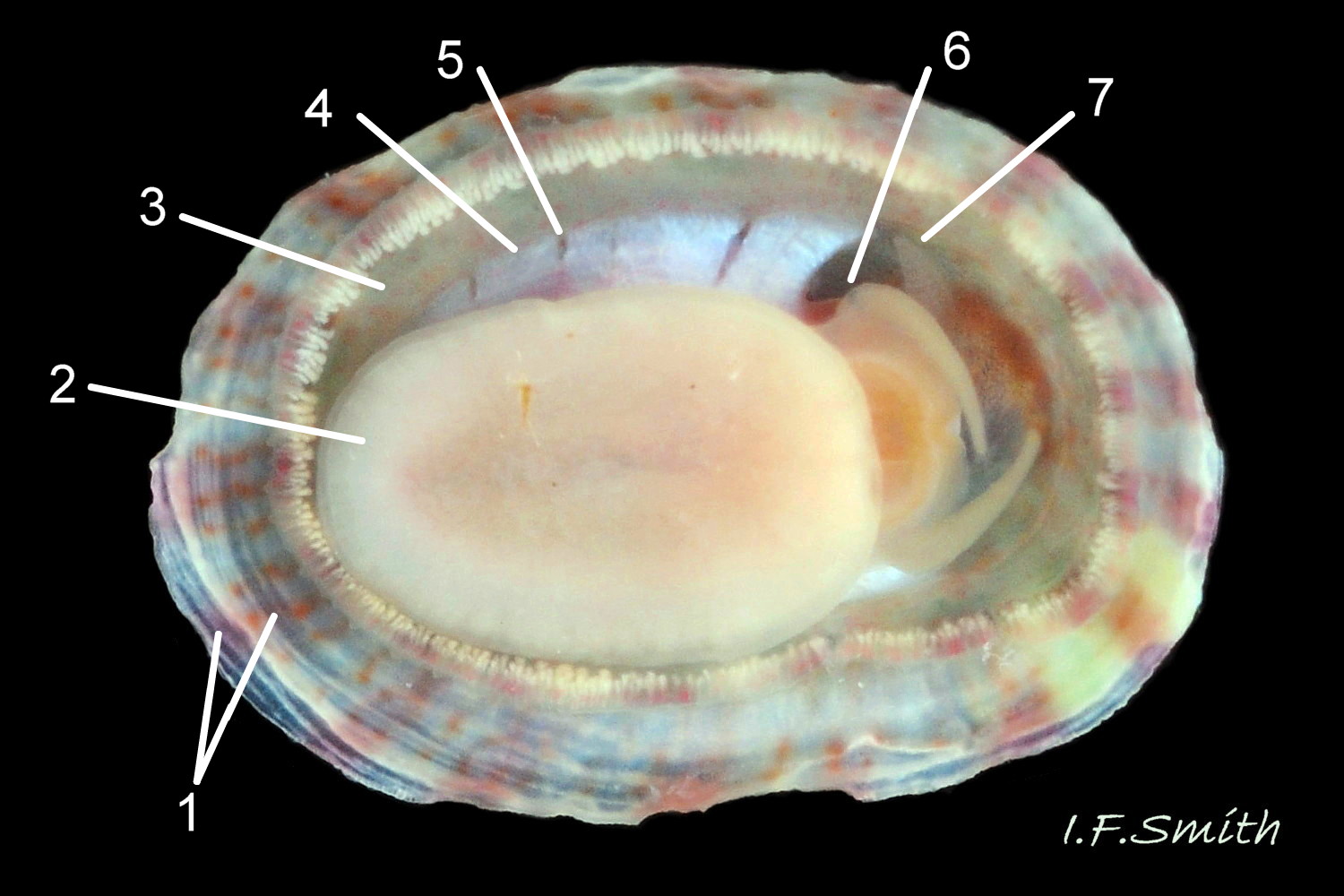
Anterior profile almost flat with small shallow concavity that diminishes when adjacent tilted apex is eroded; posterior profile flat to slightly convex 03 Testudinalia testudinalis. Superficially smooth, but major growth lines, and many finer ones, run concentrically around the shell 05 Testudinalia testudinalis; spacing between them is closer on the anterior as growth is more rapid at the posterior. Consequently, the lines, seen from the side, have a downward tilt towards the anterior 06 Testudinalia testudinalis. Many fine ridges radiate from the apex, though they are often eroded near it. The visibility of the radiating lines and growth lines of an individual shell varies with the viewing conditions, and they may be eroded from some or most of a shell 07 Testudinalia testudinalis. Shell matt, opaque or slightly translucent. External ground colour of live, clean shell lacking epizoic growths is whitish; sometimes slightly darkened or greenish if shell translucent enough to transmit colour of shell interior or mantle 08 Testudinalia testudinalis. Brown and blackish brown marks radiate from the apex, bifurcating and reuniting to form a reticulated pattern that may be a wide open net with the white ground colour dominant 09 Testudinalia testudinalis, but, very often, brown predominates forming a tessellation of approximately rectangular, brown marks 10 Testudinalia testudinalis which may merge to create a generally brown shell 11 Testudinalia testudinalis. Shell may be colourless transparent showing the green mantle and dark shell interior, occasionally on adults 12 Testudinalia testudinalis and frequently on small juveniles 13 Testudinalia testudinalis. Shell colour of live specimens may be affected by closely adhering thin coating of brown or green algae 14 Testudinalia testudinalis. Colours are less bright on dry shells, but, usually, growth lines are more distinct. Internally, shell 04 Testudinalia testudinalis has 1) an aperture rim coloured as exterior; secreted by the yellow to mustard brown mantle-edge 15 Testudinalia testudinalis, 2) a wide, matt white, peripheral zone 16 Testudinalia testudinalis; secreted by the green mantle-skirt, 3) a very thin, whitish mantle-attachment scar 17 Testudinalia testudinalis, most distinct at the anterior where it is not flanked by 4) the narrow, glossy, white U-shape pedal-retractor muscle scar, 5) an amphora shaped, chocolate brown area enclosed by scars 3 & 4; secreted by green mantle over visceral hump and 6) a cream/orange/pale brown patch at the vertex.
Body description
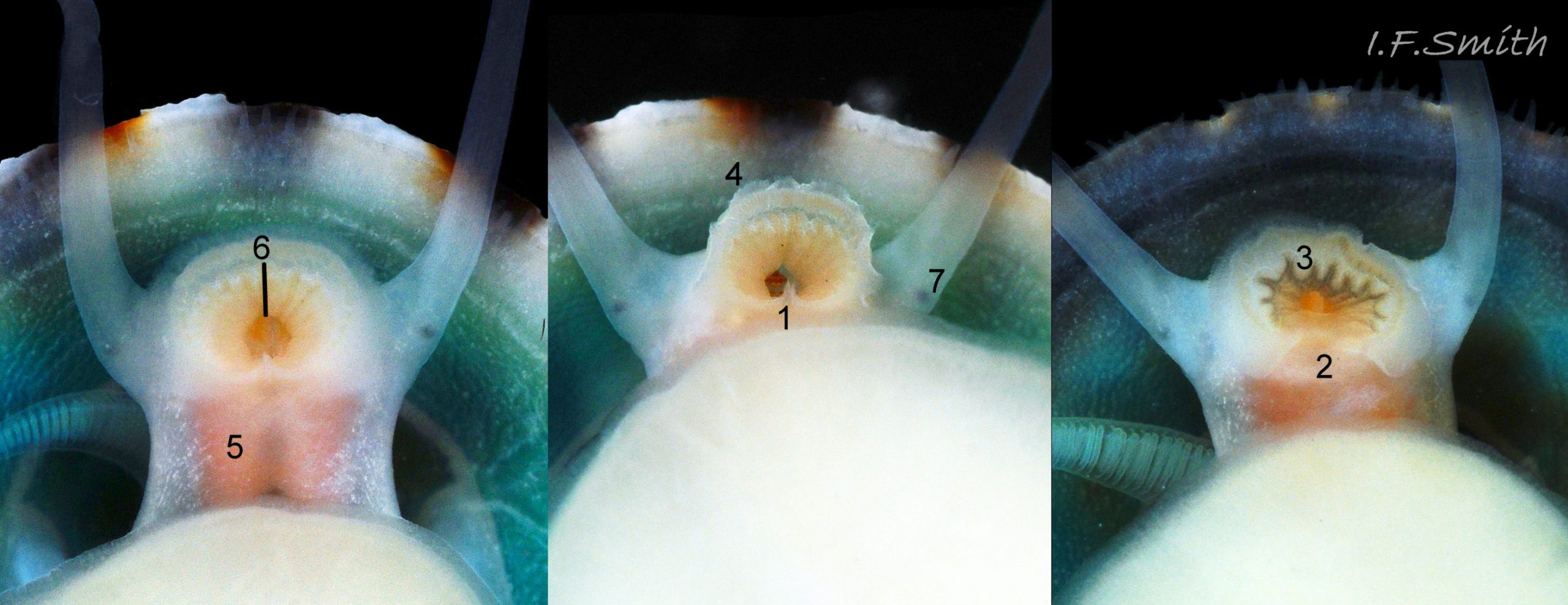
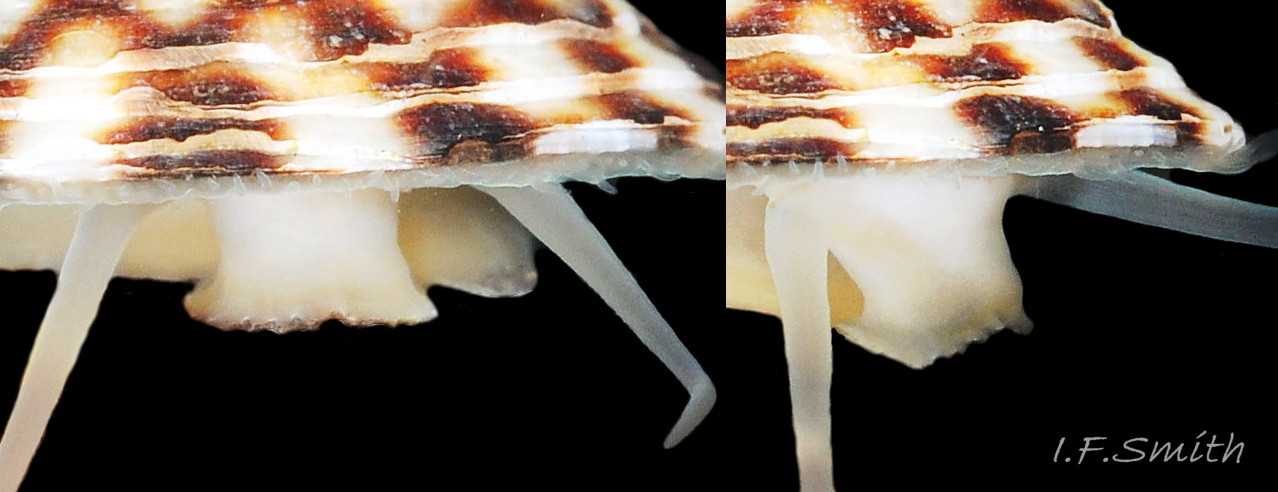
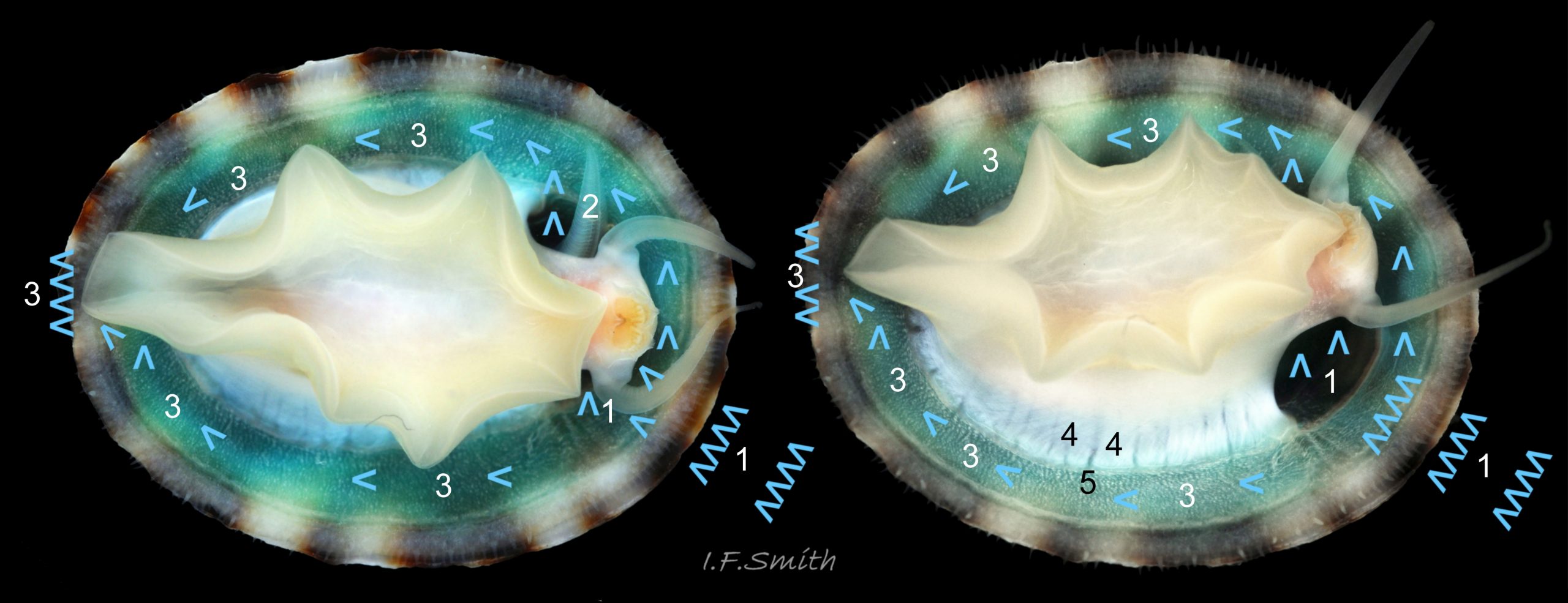
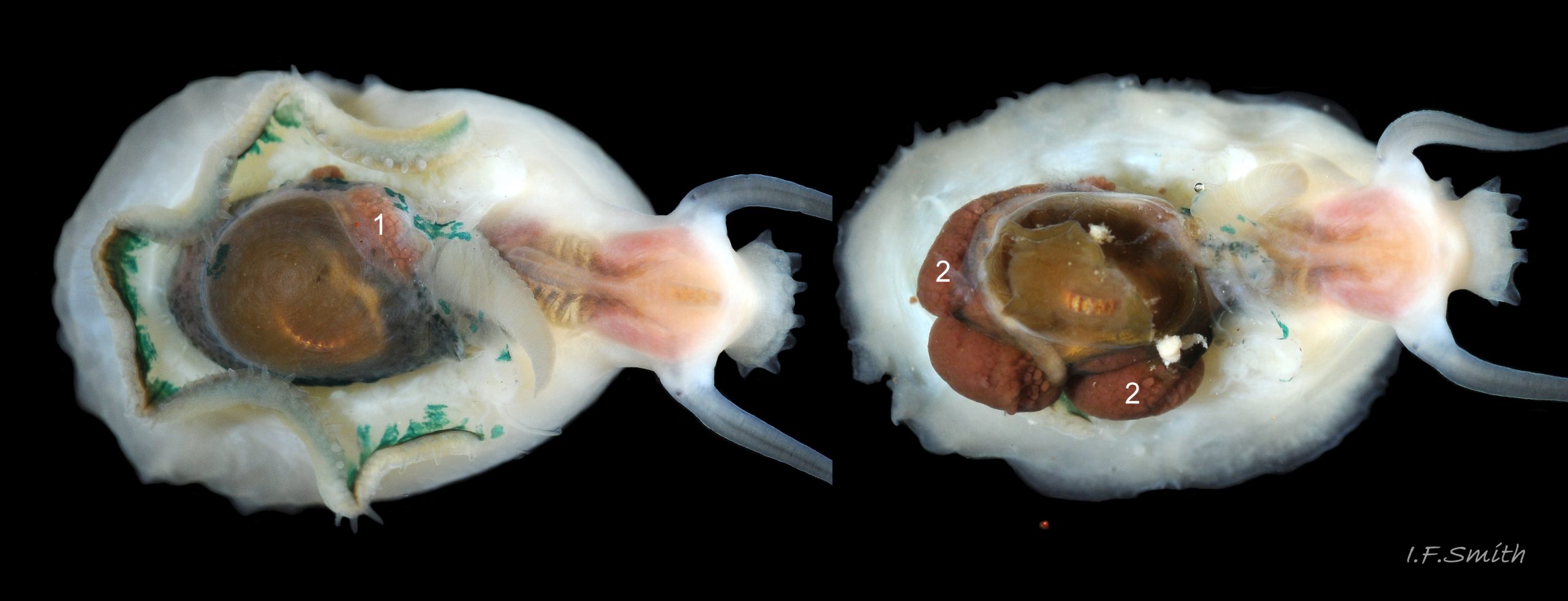
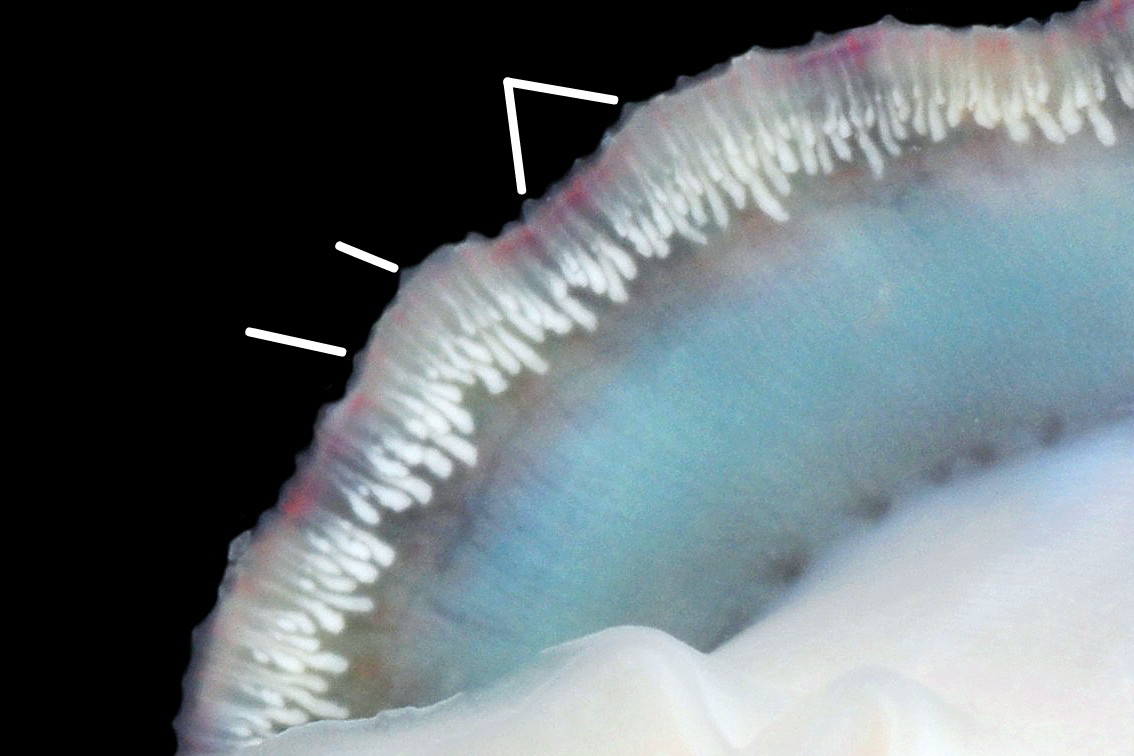
Main colour of flesh is white or yellowish white 18 Testudinalia testudinalis. Head has short stout snout with a large mouth. Large,extendible, outer lips have a gap ventrally which can be held closed or open 19 Testudinalia testudinalis. The lips can be held in a variety of positions, sometimes resembling a large snout 20 Testudinalia testudinalis & 21 Testudinalia testudinalis. The lips are thin and flimsy when extended, but are reinforced internally by longitudinal ribs that, when held together, give the lips a wavy edge 19 Testudinalia testudinalis. A pink, internal odontophore can be seen through the translucent, white head 19 Testudinalia testudinalis & 18 Testudinalia testudinalis. The odontophore is separated from the outer lips by the yellowish inner lips that open laterally and close to a vertical line . When they open, the radula with rust-coloured iron rich teeth is protruded. The long, slender, white, unpigmented cephalic tentacles are up to 70% of shell length when fully extended 20 Testudinalia testudinalis. The tiny eye on the base of each tentacle dorsally is a deep, narrow pit, detectable in the translucent tentacle as a black line running in to a broader black spot 18 Testudinalia testudinalis. When viewed ventrally through the tentacle, the broad internal spot is faintly discernible 19 Testudinalia testudinalis. Some or all of the black may be pigmented retinal cells. The eye can probably differentiate light from shade, and detect the direction of the light source, but cannot discern shapes. The mantle is translucent and colourless but usually covered with a dense layer of removable pigment; emerald green apart from a peripheral mustard brown border 15 Testudinalia testudinalis. When viewed ventrally on a live specimen, only the mantle skirt is visible and the colours are affected by the adjacent shell interior and by being seen through the mantle, so the emerald green pigment often looks blue-green, and the periphery shows the dark shell through translucent white 17 Testudinalia testudinalis. The mantle skirt contains the peripheral efferent pallial vessel 22 Testudinalia testudinalisand is fringed with translucent, white pallial tentacles 23 Testudinalia testudinalis. The mantle cavity consists of a nuchal cavity over the head, and a wide pallial groove around the entire periphery of the foot-head. Unlike patellid limpets, it has no pallial gills in the pallial groove, but does have a large, extendible, bipectinate ctenidium attached to the left of the nuchal cavity that, when extended, projects from the right of the nuchal cavity 24 Testudinalia testudinalis & 25 Testudinalia testudinalis. The lamellae on the right of the ctenidium are large, but those on the left are small and often hidden from view, so the ctenidium may appear monopectinate. The pedal-retractor muscle, a U of white muscle bundles separated by narrow gaps, attaches the body/foot to the shell 26 Testudinalia testudinalis & 18 Testudinalia testudinalis. Sole and sides of foot white or pale yellowish white. Sole approximately circular when fully spread 22 Testudinalia testudinalis . Sides of foot lack features such as epipodial tentacles. When crawling, usually only the extended pallial tentacles, cephalic tentacles and, occasionally, the ctenidium protrude beyond the shelter of the shell 23 Testudinalia testudinalis. No penis as fertilization is external.
Internal functional anatomy
Blood circulation and respiration
Image links: 22 Testudinalia testudinalis, 24 Testudinalia testudinalis, 25 Testudinalia testudinalis.
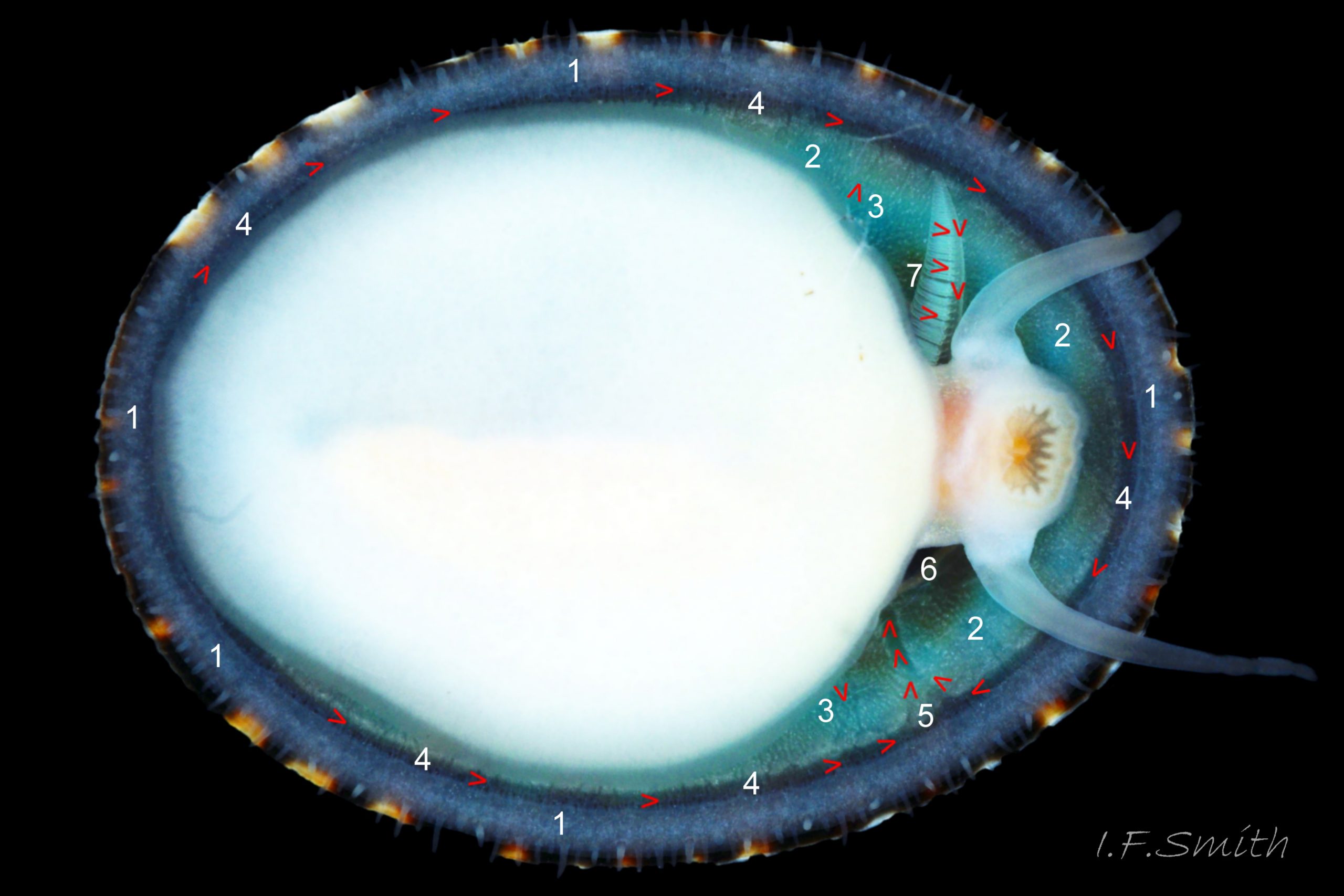
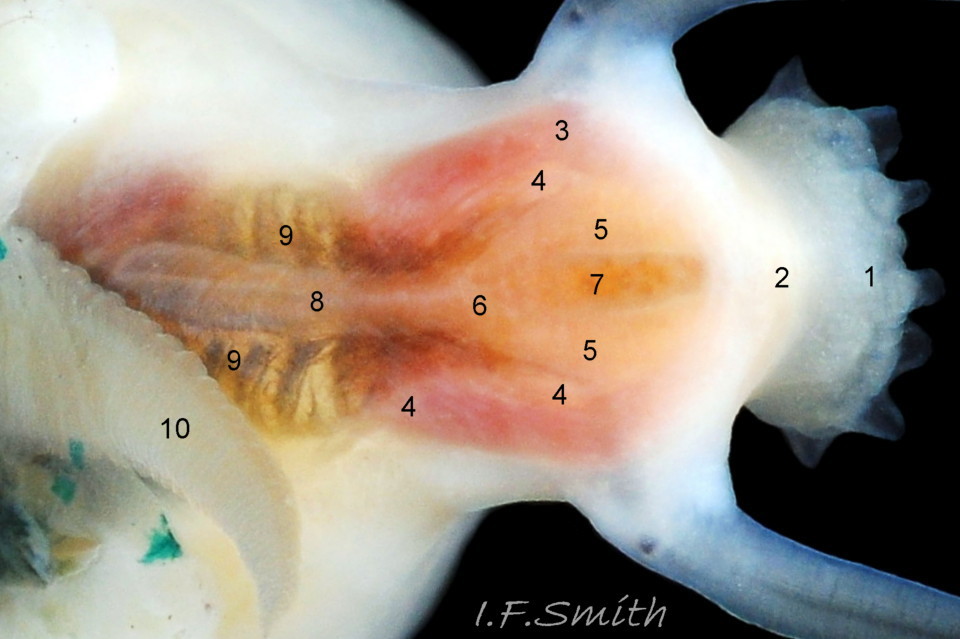
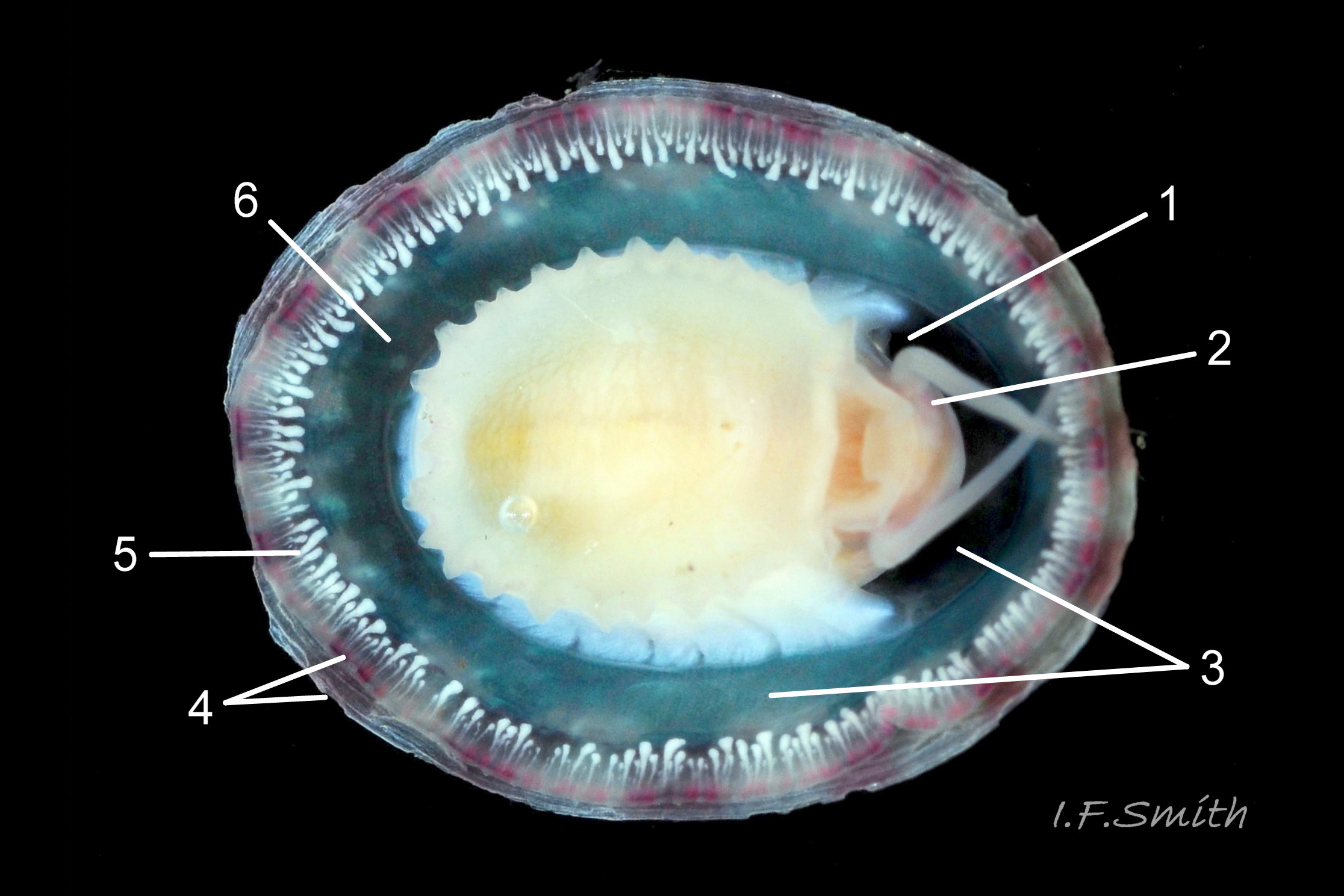
Small vessels carry oxygen-depleted, colourless blood from the visceral mass through gaps between the muscle bundles of the pedal retractor muscle ( ) and through the green mantle skirt ( ) to the efferent pallial vessel.
The large, peripheral, efferent pallial vessel ( & ) carries oxygen-depleted blood round the entire animal between zones 1 & 2 of the mantle skirt.
Blood in the efferent pallial vessel travels forwards on both sides, that on the right passes round in front of the head to the left where two vessels () carry the blood into the nuchal cavity where it passes through the ctenidium ( ) to be oxygenated and then recirculated to the body.
The green ctenidium is attached within the left of the nuchal cavity . When the limpet is in motion, the ctenidium protrudes from the right of the cavity() and may extend beyond the rim of the shell. At rest, it often contracts 50% and is concealed within the cavity. It consists of a substantial axis ( & ) with many lamellae ( & ) resembling the teeth of a comb attached to each side; bipectinate arrangement. The lamellae on the right of the axis are large ( & ), but those on the left are small (), restricted to the distal part of the axis, and often hidden from view so the ctenidium may appear monopectinate .
The inhalant current of oxygen-bearing seawater enters the nuchal cavity from the left 26 Testudinalia testudinalis and passes between the lamellae which absorb the oxygen for blood flowing through them. The oxygenated blood flows into the large branchial efferent vessel ( & ) in the axis and thence to the heart () near the base of the ctenidium to be recirculated through the body. Exhalant water currents pass along the pallial groove on either side of the body to exit at the mid-point of the posterior 26 Testudinalia testudinalis.
Alimentary and excretory features .
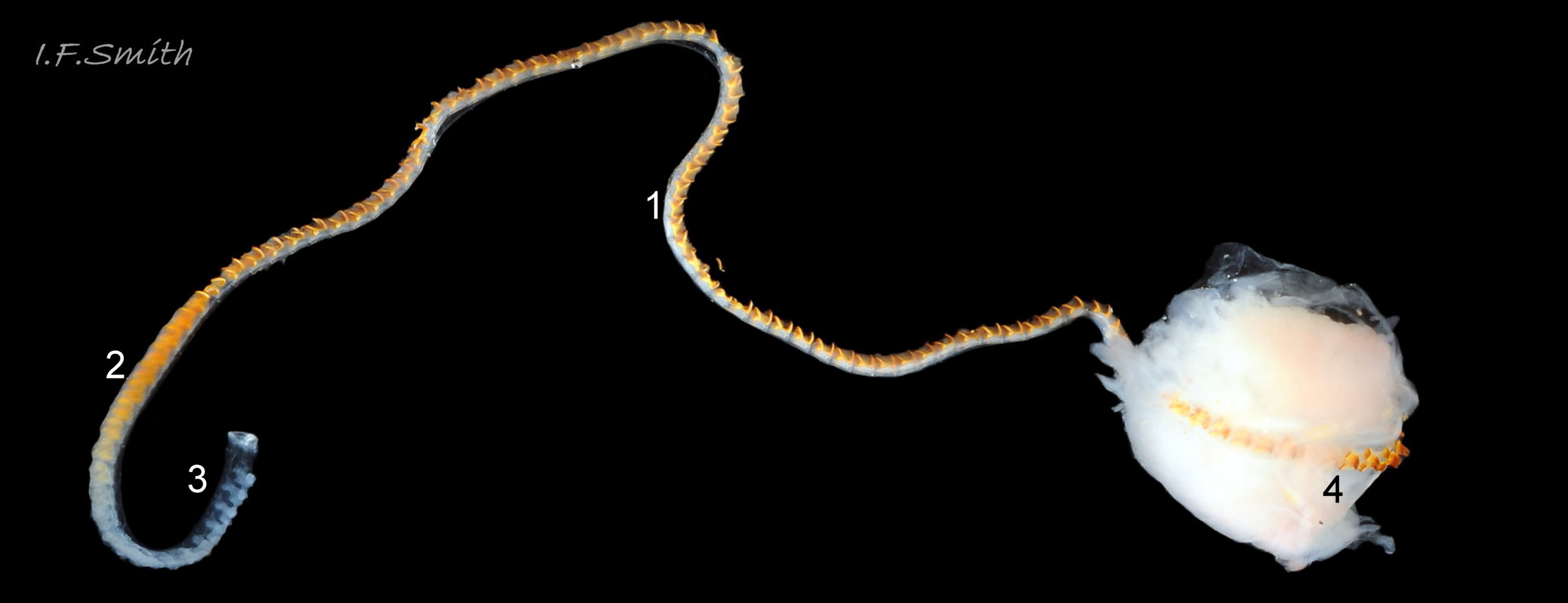
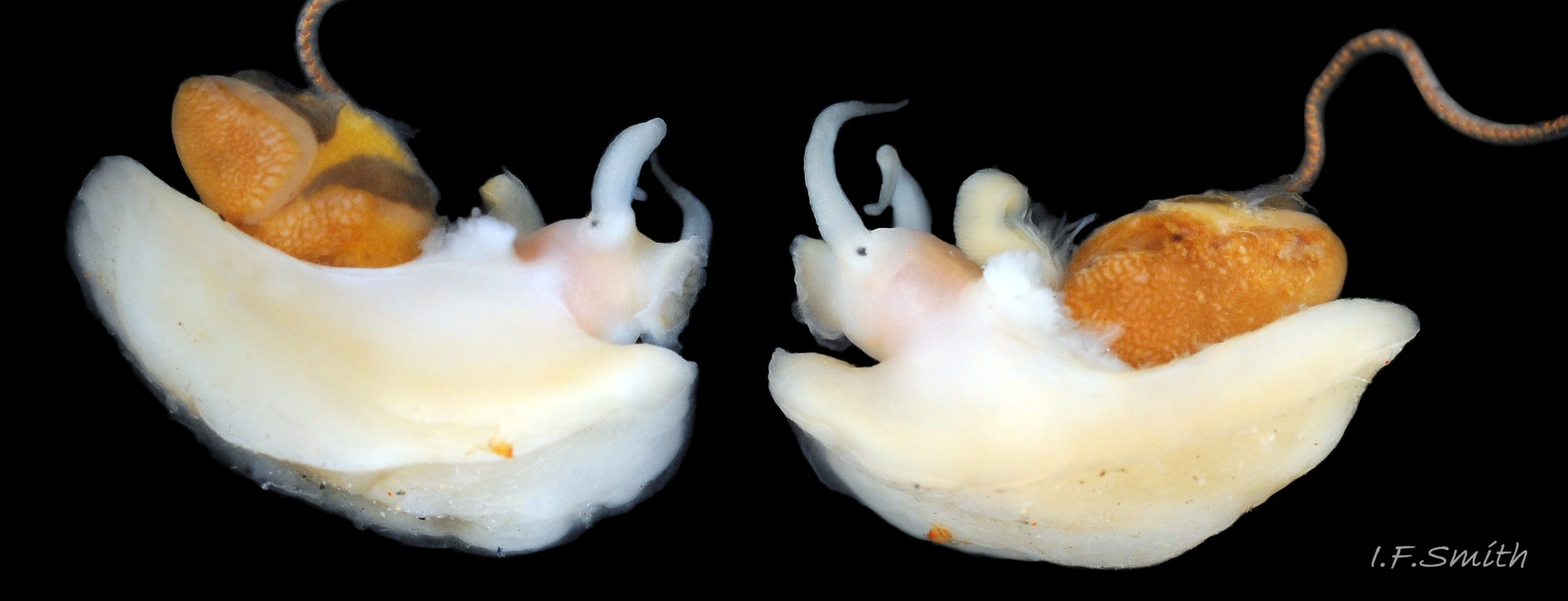
The inner lips of the mouth, described above, open into the buccal cavity. Dissection shows that the anterior wall of the cavity is reinforced by a pliable, white, chitinous, antero-dorsal plate, called the “jaw” though it is not articulated and does not bite 27 Testudinalia testudinalis. It serves as an attachment for several muscles and has two lateral wings that meet dorsally at an angle and form an anterior shield for the inner lips when they are open. Within the buccal cavity there is a large pink odontophore 27 Testudinalia testudinalis consisting of a right and left bolster which is covered in thick cuticle. Much of the bolsters is made of strong cartilage-like material.
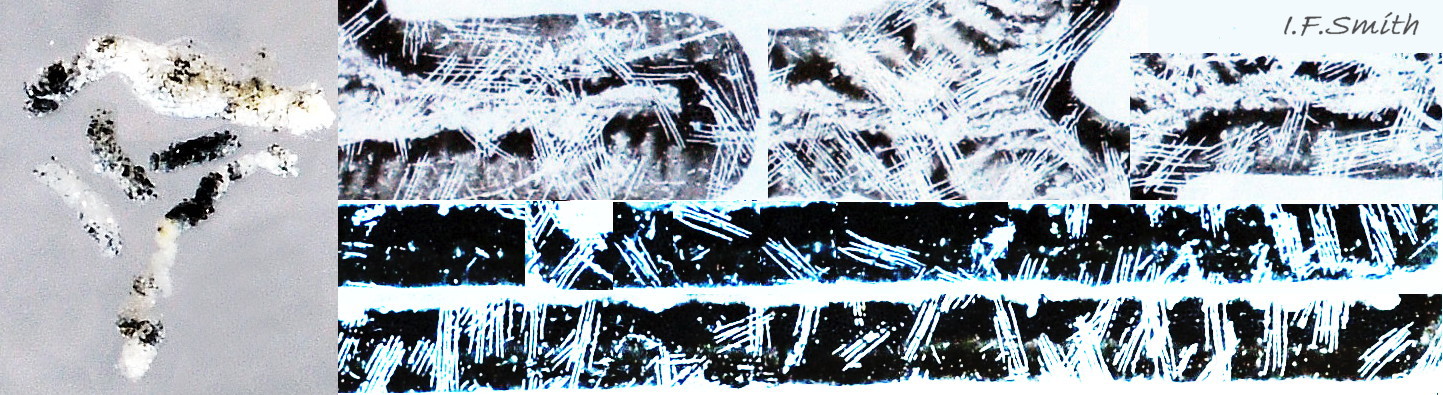
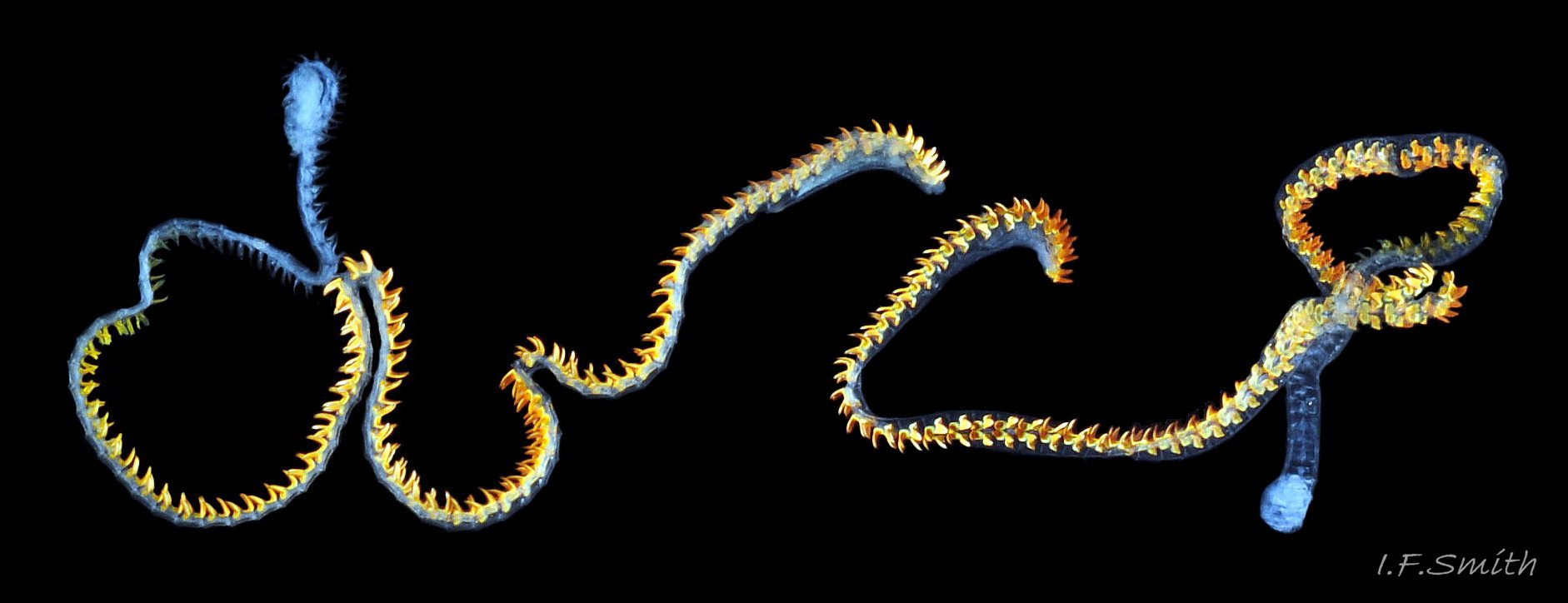
The anterior of the radula, widened into a hyaline shield, rests on the dorsum of the odontophore and is recessed slightly into the groove between the bolsters 27 Testudinalia testudinalis. The strong, iron-impregnated, unarticulated teeth are firmly fixed in a backwardly inclined position on the radula, but the anterior tip of the radula bends over the front of the odontophore so that the front row of four teeth are inclined forwards like a chisel 27 Testudinalia testudinalis. To feed, the strong muscles of the odontophore thrust it forwards against the front of the buccal cavity which, reinforced by the jaw, restrains the odontophore but allows the front teeth to project strongly from the narrow vertex of the gap between the wings of the jaw. When applied to the substrate, the teeth easily loosen diatoms, algae and other growths coating bedrock and boulders, and the curve of the withdrawing teeth acts as a scoop to lift particles back to the oesophagus in the buccal cavity. The action is surrounded by the outer lips which prevent the escape of loosened food fragments. Marks left by the front four teeth on the printed surface of a polythene sheet show that specimens with shells c. 14mm long make straight thrusts of about 0.5mm at each stroke 28 Testudinalia testudinalis. Like other limpets and some sea snails that graze rock surfaces, T. testudinalis has a radula considerably longer than its shell 29 Testudinalia testudinalis which requires several folds to fit it inside its body 30 Testudinalia testudinalis. The cause of the correlation between length and rock grazing is uncertain. It may be that a lengthy process is needed for the teeth to acquire the required hardening mineralization. Tooth creation starts at the slightly bifid, white, inner end of the long radular sac 31 Testudinalia testudinaliswith secretion of colourless transparent cuticular material by odontoblast cells. As each new tooth commences, the previous one is pushed forwards along the sac. Cells along the roof of the sac make incremental additions, including the hardening salts of iron and silicon, to the teeth as they travel along the sac, with a progressive change from colourless through darkening shades of yellow/orange visible through the translucent sac walls. Creation is complete by the time the tooth reaches the buccal cavity, where it emerges from the radular sac onto the odontophore in front of the opening of the oesophagus.
A whitish salivary duct runs next to each of the two dorsal folds of the oesophagus 32 Testudinalia testudinalis. The ducts carry mucus from the salivary gland to the buccal cavity to lubricate the feeding process and bind the food particles brought in by the radula. The mucus does not have a digestive function. In the rear of the buccal cavity, the mucus-bound food particles pass into the entrance of the oesophagus 32 Testudinalia testudinalis. and the radula passes into the radular sac directly below the oesophagus (so now out of sight in dorsal view, except for a short section usually visible at the surface of the digestive gland 33 Testudinalia testudinalis). Lubricating mucus is provided to the oesophagus by the oesophageal gland consisting of a series of tubules on either side of it 32 Testudinalia testudinalis The oesophagus passes into the visceral mass. Food is moved along it by cilia to where it widens to become the stomach. The digestive gland 33 Testudinalia testudinalis, composed of a mass of tubules and usually the most obvious organ on the surface of the visceral mass when the shell is removed, opens into the stomach through a duct. Digestive cells in the tubules ingest particulate food to digest it intracellularly (Fretter & Graham, 1994, p. 219). The tubules extend into the blood filling the haemocoel, and their very thin covering of connective tissue allows the passage of nutrients into the blood. Undigested material passes into the long coiled intestine 33 Testudinalia testudinalis where it is compressed and bound with mucus 28 Testudinalia testudinalis to prevent fouling of the ctenidium. The faecal string passes through the rectum 33 Testudinalia testudinalis to emerge from the anus at the rear right of the nuchal cavity and, with particulate matter removed by cilia from the inhalant water, is conveyed along the pallial groove by cilia, helped by the flow of exhalant water, to be expelled from the mid-point of the posterior of the shell 26 Testudinalia testudinalis. The large right nephridium ( kidney) is attached to the inner surface of the mantle 34 Testudinalia testudinalis, but often difficult to distinguish when it is the same colour as the viscera below it 33 Testudinalia testudinalis. The right nephridium extends round onto the left of the animal. The nephridipores (openings) of it and the smaller, unobtrusive, left nephridium are sometimes visible 34 Testudinalia testudinalison either side of the anus . Their urogenital products are conducted to the posterior of the animal by cilia and the exhalant water current.
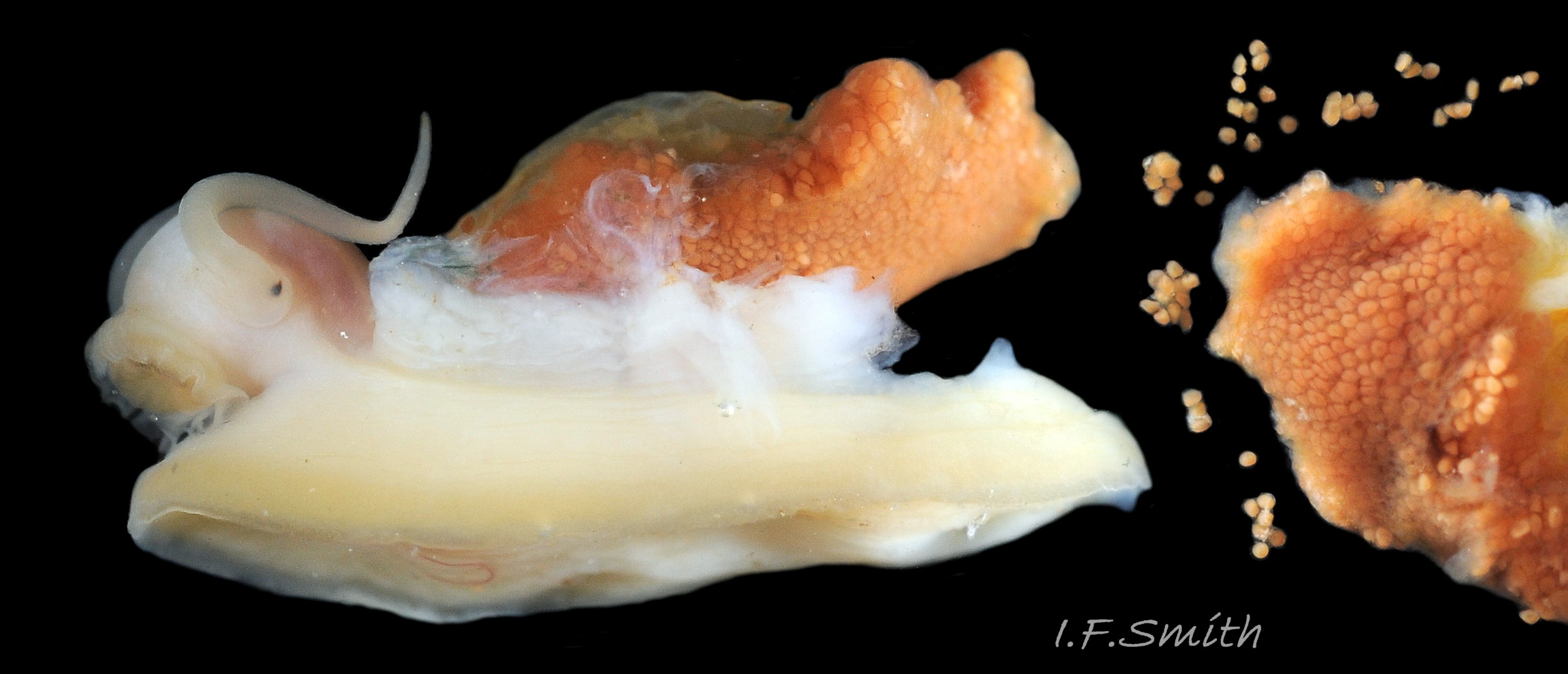
Reproductive organs
The gonads are situated between the viscera and foot. Just before and during breeding, they may spread over much of the viscera, but they are usually hidden, apart from a small section, on an animal removed from the shell. If the mantle is taken off a female, her ovaries may rapidly expand as the ova absorb water and the constraint of the mantle is removed 35 Testudinalia testudinalis. Female ovaries are granular, and individual red/brown ova readily break away 36 Testudinalia testudinalis . Male testes have numerous interconnected tubules 37 Testudinalia testudinalis. Male and female gonads have similar orange-brown colours, or pink (Fox, 2003), with individual variation of shade which may change with breeding condition. Fertilization is external, so the male has no penis. Gametes leave both sexes through the right nephridium (kidney) via its nephridipore 34 Testudinalia testudinalis close to the anus in the nuchal cavity. The female also exudes a thin mucous film that secures the ova to the substrate.
Key identification features
Testudinalia testudinalis
1: Maximum length usually 20mm, occasionally 25mm, rarely 30mm.
2: Shell exterior matt-whitish with radiating chocolate-brown rays that often bifurcate and reunite across the shell 01 Testudinalia testudinalis
3: Shell interior porcelaneous-white with brown-banded peripheral border, an amphora-shaped, chocolate-brown patch and a pale vertex patch 04 Testudinalia testudinalis.
4: No prominent sculpture (except sometimes irregular repair-line of damage), but many fine concentric growth lines and radiating striae 05 Testudinalia testudinalis.
5: Mantle skirt with emerald green pigment dorsally that looks blue-green when viewed ventrally 17 Testudinalia testudinalis.
6: Large pallial tentacles protrude beyond shell perimeter when active 23 Testudinalia testudinalis.
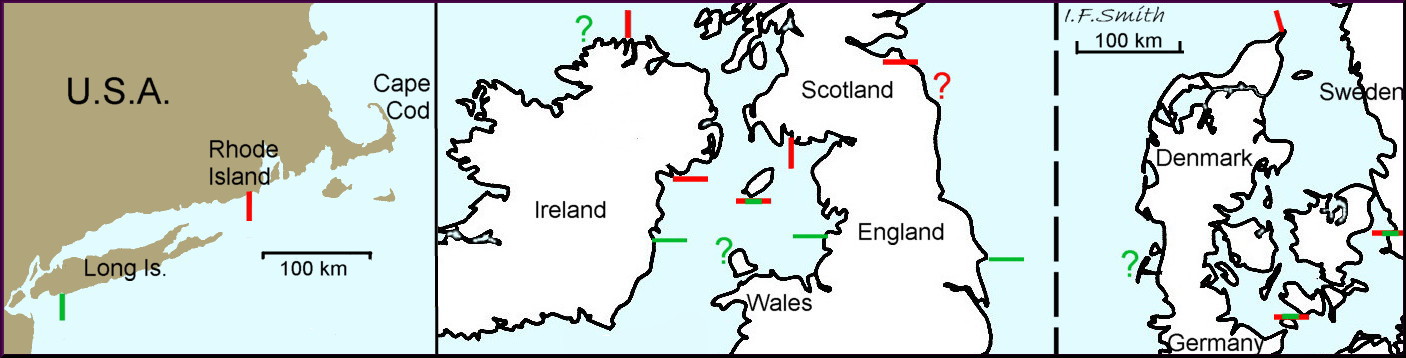
7: Northern species stretching south to southern Scotland, northern Ireland, Isle of Man, south-west Sweden and Rhode Island, USA.
8: Sometimes, but more often not, on pink, calcareous, encrusting algae with no pale feeding pits, and faecal rods with flat truncated ends that are not chalk-white.
Similar species
Tectura virginea
The only ‘tortoiseshell limpet’ in the southern half of Britain where it is often mis-recorded as Testudinalia testudinalis.
1: Maximum length 12mm.
2: Shell exterior whitish/yellowish/bluish with radiating pinkish rays and/or light-brown chains which are often mistaken for brown marks of T. testudinalis, especially small juveniles 42 Testudinalia testudinalis.
3: Shell interior white often translucent showing exterior marks, sometimes red-brown V near vertex 43 Testudinalia testudinalis
4: Sculpture of slight threadlike radiating striae 43 Testudinalia testudinalis, often indistinct or absent 44 Testudinalia testudinalis , and numerous fine concentric growth lines
5: Mantle-skirt white, yellowish, or blue-green with reddish bands on periphery 45 Testudinalia testudinalis, 46 Testudinalia testudinalis . and 47 Testudinalia testudinalis.
6: Outer edge of mantle has many small, unobtrusive, translucent processes and many large white repugnatorial glands pointing inwards from mantle edge, but no prominent outward pointing pallial tentacles 48 Testudinalia testudinalis.
7: All round Britain except Liverpool Bay and parts of SE England.
8: Nearly always on pink, calcareous, encrusting algae with pale feeding pits and short, chalk-white faecal rods with hemispherical ends.
Habits and ecology
T. testudinalis occurs on rocky shores at MLWST, or MHWNT in pools, and to 50m depth. It feeds on diatoms and algae 12 Testudinalia testudinalis coating bedrock and boulders. Eulittoral specimens are often found stationary on bare vertical 38 Testudinalia testudinalis or overhanging 39 Testudinalia testudinalis surfaces during daylight hours. Fretter and Graham (1962) say it and Tectura virginea feed on encrusting algae but, though T. virginea is usually found on/near it, only 18 of 300 images of T. testudinalis on iNaturalist show encrusting algae. Lord (2008) showed that immersed laboratory specimens fed nocturnally on alga-encrusted rocks but in daylight moved onto bare vertical rocks, where they remained stationary. A homing instinct, if any, is weakly developed in this species, so a home scar is not engraved into soft rock (Lord, 2008).
Defence: the simple eyes 18 Testudinalia testudinalis may be able to detect the shadow of an attacker, but the principal warning organs are probably the touch-sensitive, long cephalic tentacles, plentiful encircling pallial tentacles 23 Testudinalia testudinalis and large outer lips 20 Testudinalia testudinalis. The white and brown tessellated shell is probably cryptic on the bare rocks favoured in daylight 08 Testudinalia testudinalis. Predators include gulls, crabs and starfish. When threatened by a starfish, it exhibits, like small Patella vulgata, a flight response (Lord, 2008).
T. testudinalisbreeds in spring and early summer (April to July in New England). Gonochoristic; the male lacks a penis, but he mounts a female’s shell, sometimes for several hours in expectation, to be proximate when she spawns so he can release his sperm to be drawn in by her inhalent current as the eggs emerge 40 Testudinalia testudinalis.. In a refrigerator maintained at about 8ºC, some of a group of eight individuals, collected in late May, bred in June producing a thin film of mucus with the ova widely spaced and attached to it (pers. obs.). The film was only loosely attached to the smooth base of the container, but adhesion would probably be better on a rough surface. The eggs hatched by late June as free trochophore larvae (stage passed within egg by most less “primitive” spp.) but further development failed. In the wild, after a short time in the plankton, the trochophores metamorphose into veligers and, after further growth in the plankton, settle and assume limpet form by August with a thin, translucent, 2 mm long shell 13 Testudinalia testudinalis..
Distribution and status
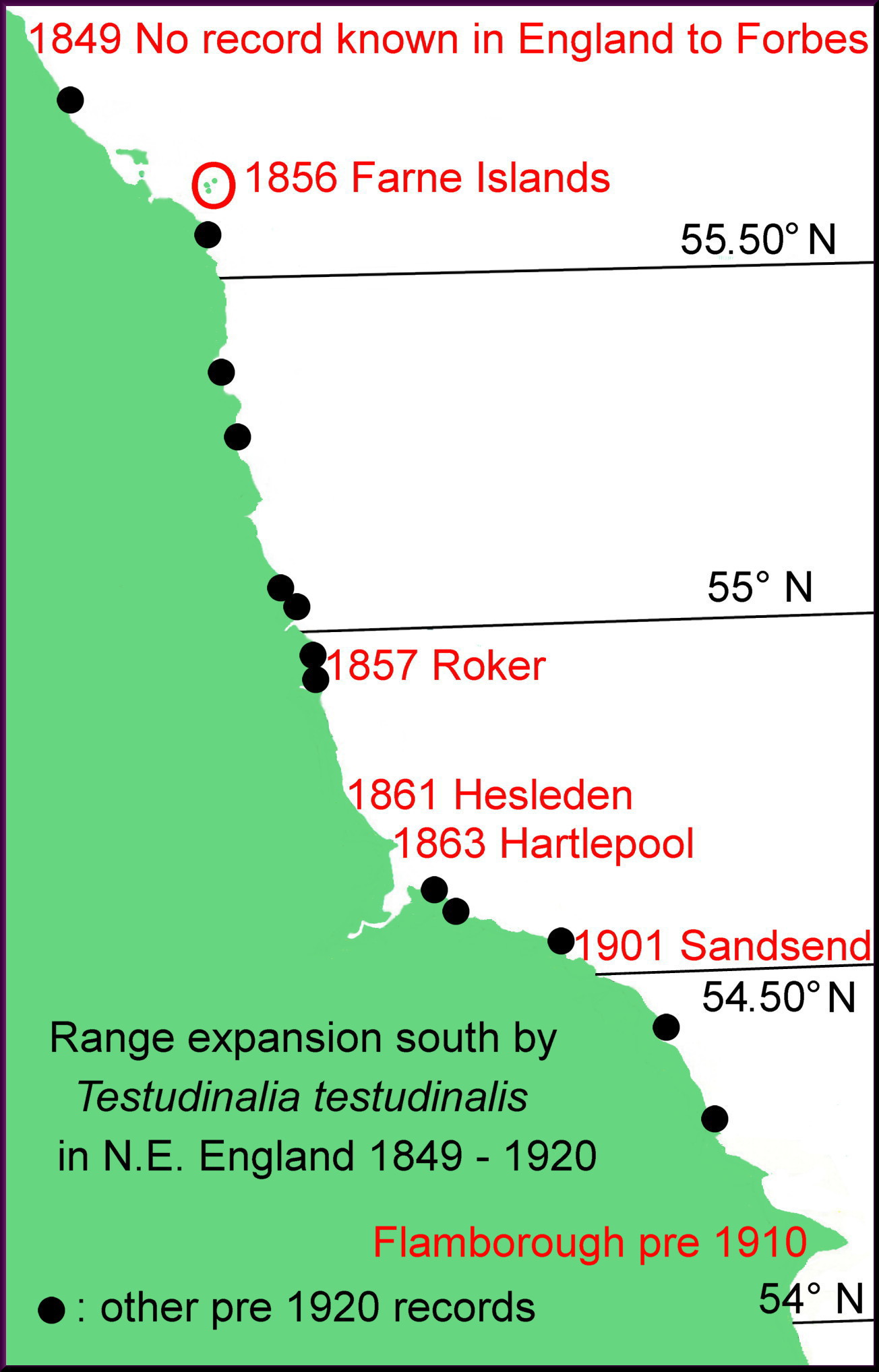
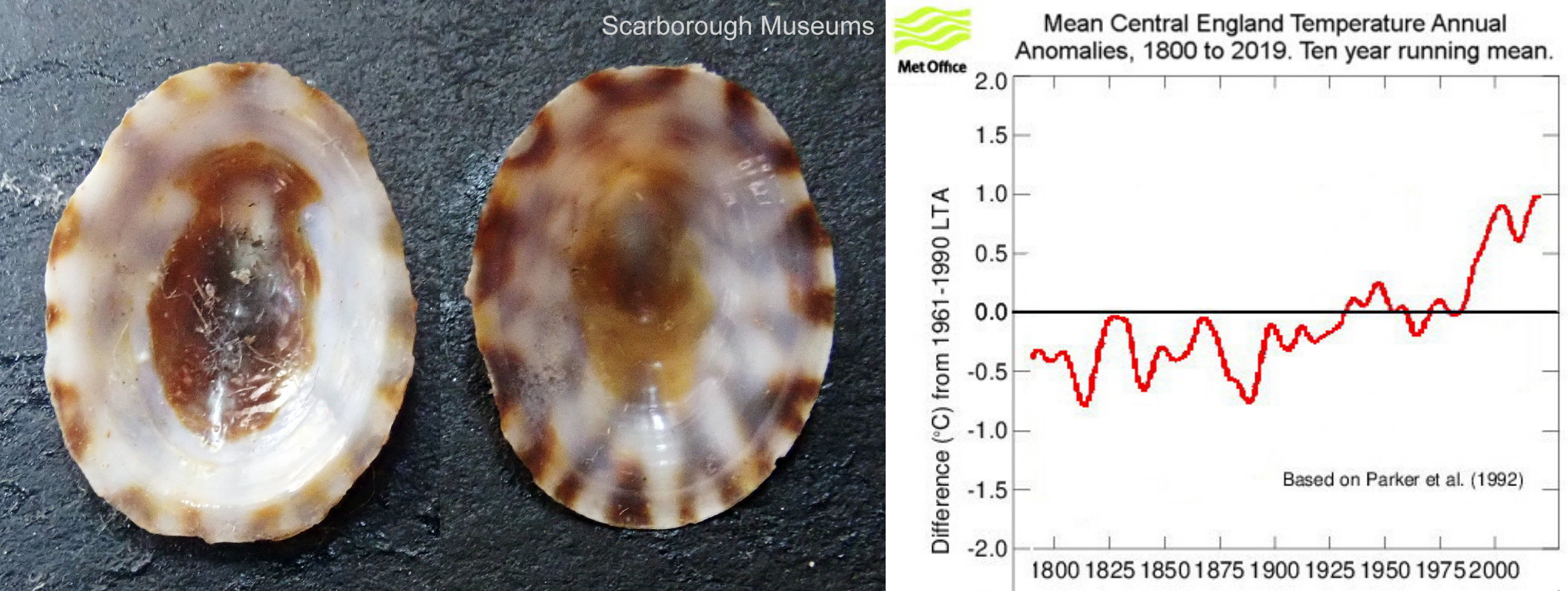
T. testudinalis is a cold-water, northern species in Arctic Canada, Greenland, Iceland and northern Russia, which extends south to Rhode Island (USA), Britain, Ireland, southern Scandinavia and the German Baltic, but not the brackish inner Baltic. In the relatively cold 19th century is extended its range southwards in Britain, Ireland and probably elsewhere, except the Baltic and Gulf of St Lawrence where low salinity probably prevented its spread. In the late 20th century and early 21st century, when temperatures rose markedly at an increasing rate, its distribution seems to have fallen back northwards to about its pre-spread positions.
The appendix at A02 describes in detail the distribution limits at different dates.
Acknowledgements
I am indebted to Simon Taylor and David W. McKay for providing me with specimens for photography and study. The account would not have been possible without their help. I thank Dr Paula Lightfoot and Becky Hitchin for generously providing images and information. The much valued advice of Dr Julia Sigwart and Dr Lauren Sumner-Rooney is gratefully acknowledged. Many thanks also to Inga Williamson for shore work related to the account.
A separate acknowledgement is made after the Appendix at A02 of those who contributed to its creation.
Links and references
Forbes, E. & Hanley S. 1849-53. A history of the British mollusca and their shells. vol. 2 (1849), London, van Voorst. (As Acmaea testudinalis; Free PDF at archive.org/stream/historyofbritish02forb#page/434/mode/2up Use slide at base of page to select pp.434-437.)
Fox, R. 2003. Invertebrate Anatomy On Line; Tectura testudinalis
lanwebs.lander.edu/faculty/rsfox/invertebrates/tectura.html
Fretter, V. and Graham, A. 1962. British prosobranch molluscs. London, Ray Society.
GBIF (Global Biodiversity Information Facility). Distribution map for T. testudinalis accessed October 2020. Includes many obvious errors such as far-inland or tropical locations. www.gbif.org/species/4369953
Graham, A. 1988. Prosobranch and pyramidellid gastropods. London.
Hargreaves, J. A. 1910. The marine mollusca of the Yorkshire coast and the Dogger Bank. J. Conch., Lond. 13: 80 – 105.
iNaturalist map and images of T. testudinalis records (accessed October 2020) www.inaturalist.org/taxa/415169-Testudinalia-testudinalis
Jeffreys, J.G. 1862-69. British conchology. vol. 3 (1865). London, van Voorst. (As Tectura testudunalis; Free PDF at archive.org/stream/britishconcholog03jeff#page/246/mode/2up . Use slide at base of page to select pp.246- 248.
Lebour, M.V. 1902. Marine mollusca of Sandsend. The Naturalist.
Lord, J. 2008. Movement patterns and feeding behaviour of the limpet Tectura testudinalis (Müller) along the mid-Maine Coast Honors Theses. Paper 243. digitalcommons.colby.edu/honorstheses/243
Lumb, F.E. 1961. Seasonal variation of the sea surface temperature in coastal waters of the British Isles. Scientific paper no. 6. London, HMSO.
pdf at digital.nmla.metoffice.gov.uk/file/sdb%3AdigitalFile%7Cc2…
McMahon, R. F. & Russell-Hunter, W. D. 1977. Temperature relations of aerial and aquatic respiration in six littoral snails in relation to their vertical zonation. Biol. Bull. 152: 182 to198.
Publication info: Woods Hole, Mass. :Marine Biological Laboratory,
View at www.biodiversitylibrary.org/page/1540015#page/202/mode/1up
whole bulletin is 38 MB, but page has option to create 5MB pdf of just the article by selecting pp 182 to 198
www.biodiversitylibrary.org/pdf4/066873200017332.pdf
Mieszkowska, N., Leaper, R., Moore, P., Kendall, M.A., Burrows, M.T, Lear, D., Poloczanska, E., Hiscock, K., Moschella, P.S., Thompson,R.C., Herbert, R.J., Laffoley, D., Baxter, J., Southward, A.J., & Hawkins, S.J. 2005. Assessing and predicting the influence of climatic change using eulittoral rocky shore biota. M.B.A. Occasional publication No. 20.
www.researchgate.net/publication/281164392_Assessing_and_…
Parker, D.E., T.P. Legg, and C.K. Folland. 1992. A new daily Central England Temperature Series, 1772-1991. Int. J. Clim., Vol 12, pp 317-342 www.metoffice.gov.uk/hadobs/hadcet/ [includes update to 2020].
Walker, C.G. 1966. Studies on the jaw, digestive system, and coelomic derivatives in representatives of the genus Acmaea. In Abbot, D.P. et al (ed.). 1968. The biology of Acmaea. Veliger 11: supplement. 88 to 97.
pdf at archive.org/details/veliger111968berk
Yonge, C.M. and Thompson, T.E. 1976. Living marine molluscs. London.
Current taxonomy: World Register of Marine Species (WoRMS)
www.marinespecies.org/aphia.php?p=taxdetails&id=234208
GLOSSARY
afferent = (adj. of vessel) carrying blood etc. towards an organ.
aperture = mouth of gastropod shell; outlet for head and foot.
auricle = part of heart that receives blood from the adjacent ctenidium.
bipectinate = (See ctenidium.)
cartilages = (in gastropods) structures of tough, resilient material, histologically resembling vertebrate cartilage, embedded in tough connective tissue of left and right bolsters of the odontophore. Support and maintain shape of odontophore, and provide attachment for muscles controlling its movement.
cephalic = (adj.) of or on the head.
coll. = in the collection of (named person or institution; compare with leg.).
comminuted = reduced to minute particles or fragments.
ctenidium = comb-like molluscan gill; usually an axis with a row of filaments/lamellae on either side (bipectinate), sometimes on one side only (monopectinate).
efferent = (adj. of vessel) carrying blood etc. away from an organ.
ELWS = extreme low water spring tide (usually near March and September equinoxes).
emersed = not covered in water.
epipodial = (adj.) of the epipodium (collar or circlet running round sides of foot of some gastropods).
gonochor(ist)ic = (syn. dioecious) having separate male and female individuals, not hermaphrodite.
growth line = line, transverse to direction of shell increase, indicating aperture’s rim position during a pause in growth. Seasonal pauses create more major lines than shorter pauses such as diurnal ones.
haemocoel = blood-filled body cavity of gastropods. (Molluscs have an “open circulatory system” which includes large cavities with sluggishly moving blood that directly bathes organs.)
immersed = covered in water.
intracellularly = occurring within a cell or cells.
lamellae (of ctenidium) = plate like leaflets/filaments arranged like teeth of a comb.
leg. (abbreviation of legit) = collected/ found by (compare with coll.)
mantle = sheet of tissue that secretes the shell and forms a cavity for the gill in most marine molluscs.
monopectinate = (See ctenidium.)
MLWS = mean low water spring tide level (mean level reached by lowest low tides for a few days every fortnight; Laminaria or Coralline zone on rocky coasts).
nephridium (pl. nephridia) = cilia-lined excretory/osmoregulatory tubule (kidney).
nephridiopore = opening of nephridium (kidney) for excretion. a.k.a. nephropore, or renal pore.
nuchal cavity = cavity roofed by mantle that contains head of limpet; part of mantle cavity (remainder consists of pallial groove on each side of body).
pericardium= sac containing the heart.
periostracum = thin horny layer of chitinous material often coating shells.
protandrous hermaphrodite = sequential hermaphrodite with individuals starting as males and later changing to female.
trochophore = spherical or pear-shaped larva that swims with aid of girdle of cilia. Stage preceding veliger, passed within gastropod egg in most spp. but free in plankton for patellid limpets, most Trochidae and Tricolia pullus.
veliger = shelled larva of marine gastropod or bivalve mollusc which swims by beating cilia of a velum (bilobed flap).
ventricle = contractile part of heart that pumps blood.