Click image to enlarge with full caption. Main text below slider.
Acanthochitona fascicularis (Linnaeus, 1767)
Current taxonomy: World Register of Marine Species (WoRMS)
www.marinespecies.org/aphia.php?p=taxdetails&id=138677
A pdf of this and other species accounts can be downloaded from www.researchgate.net/profile/Ian_Smith19/research
Distribution Revised February 2022
Synonyms:Chiton fascicularis Linnaeus, 1767; Acanthochites communis Risso, 1826; Acanthochites hamatus Rochebrune, 1882;
[ Forbes & Hanley (1853) and Jeffreys (1865) probably called it Chiton discrepans Brown, and misapplied the name Chiton fascicularis Linnaeus to Acanthochitona crinita (Pennant, 1777). There is some uncertainty about which species Linnaeus called Chiton fascicularis. His specimen was from Algeria. ]
Vernacular: Grote borstelkeverslak (Dutch).
GLOSSARY BELOW
This account uses the standardised terminology for chitons proposed by Schwabe (2010). Some of Jones & Baxter (1987) alternatives are indicated in the glossary as a.k.a..
Shell Description
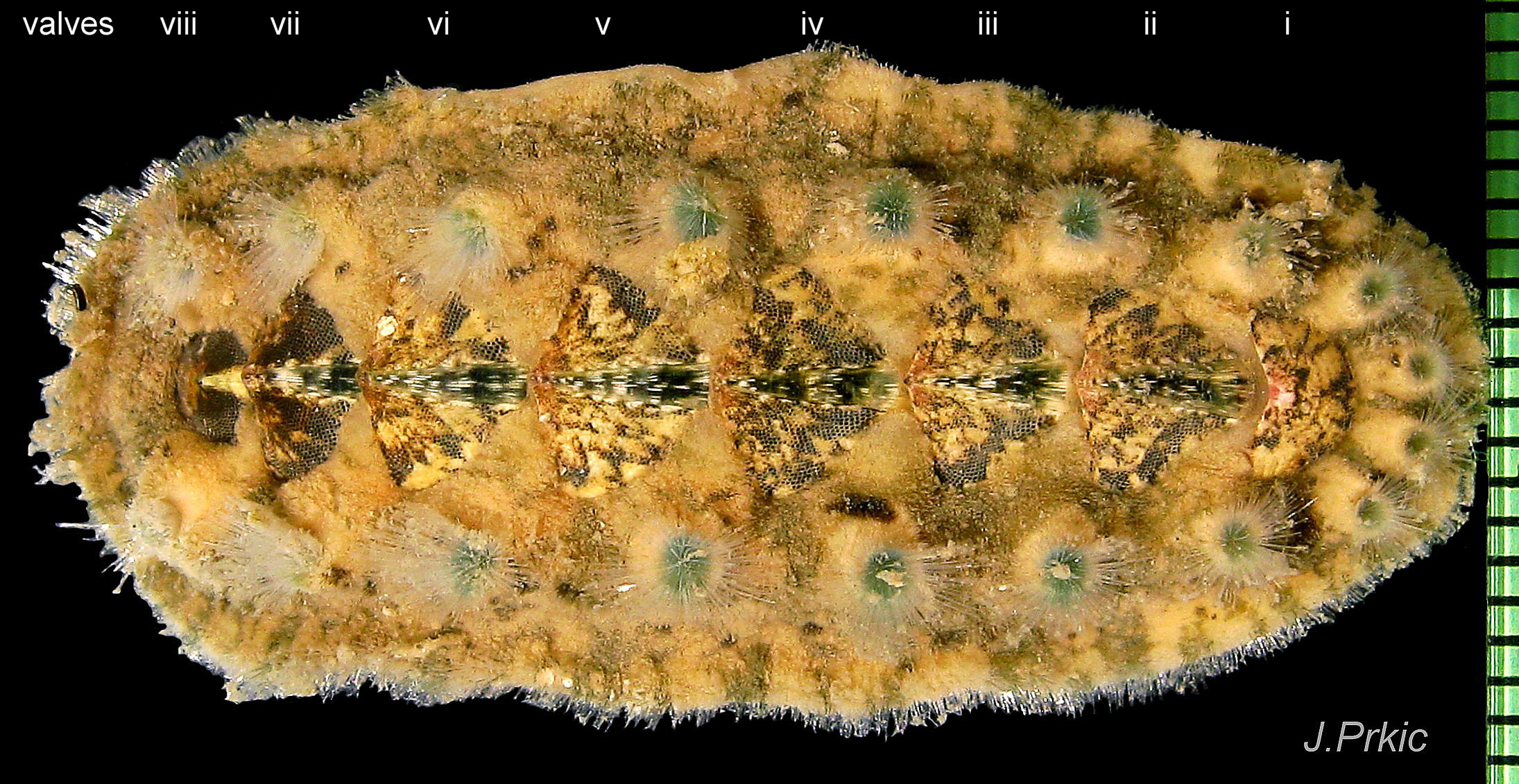
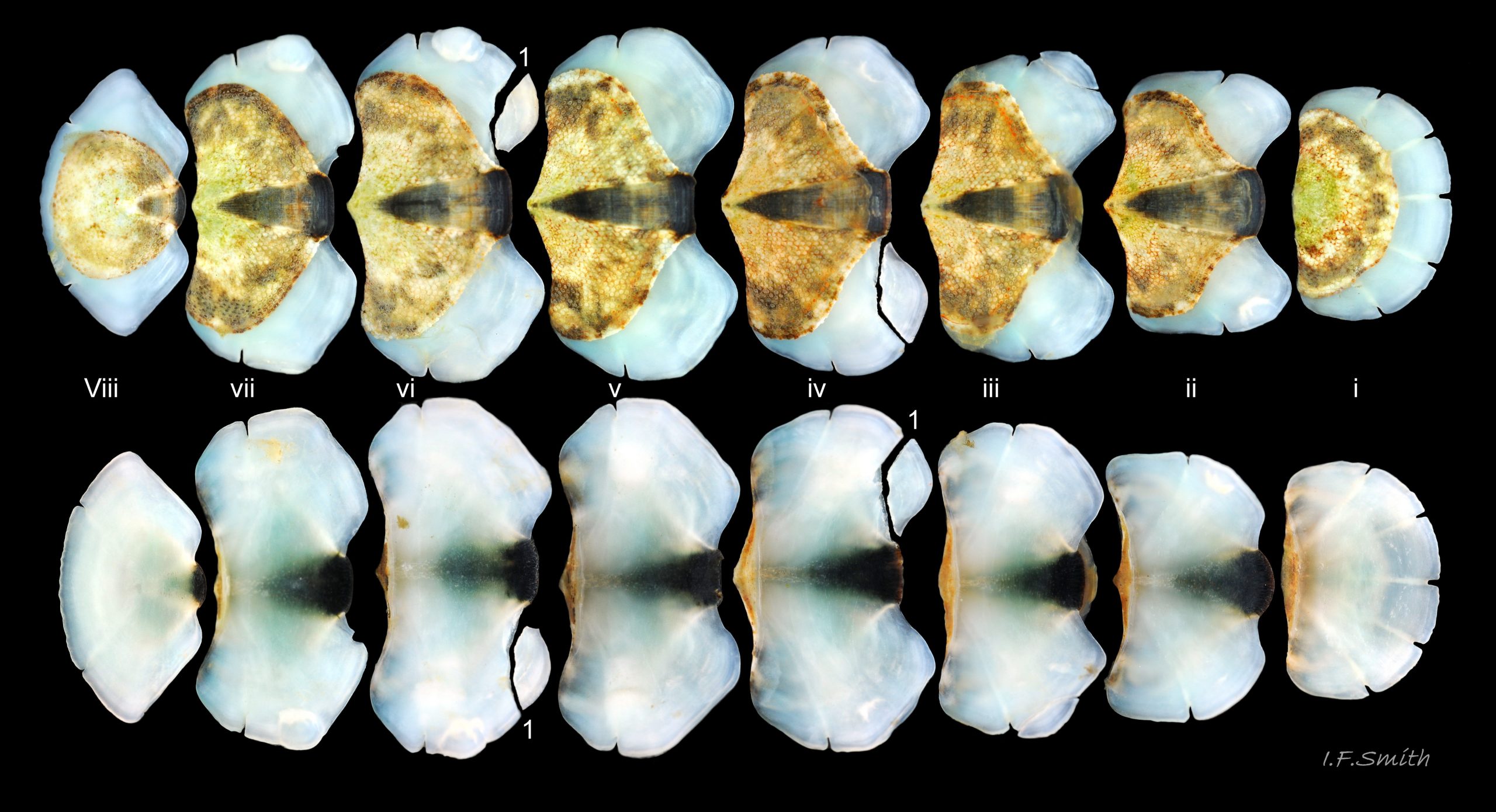
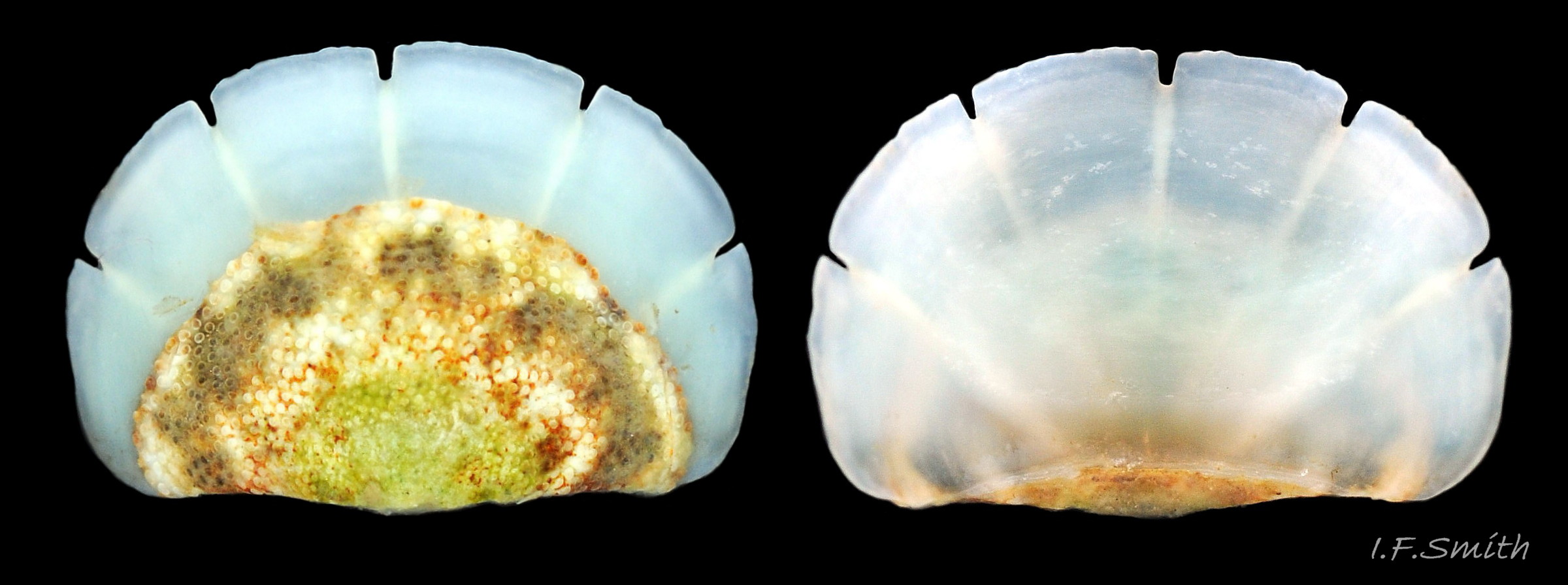
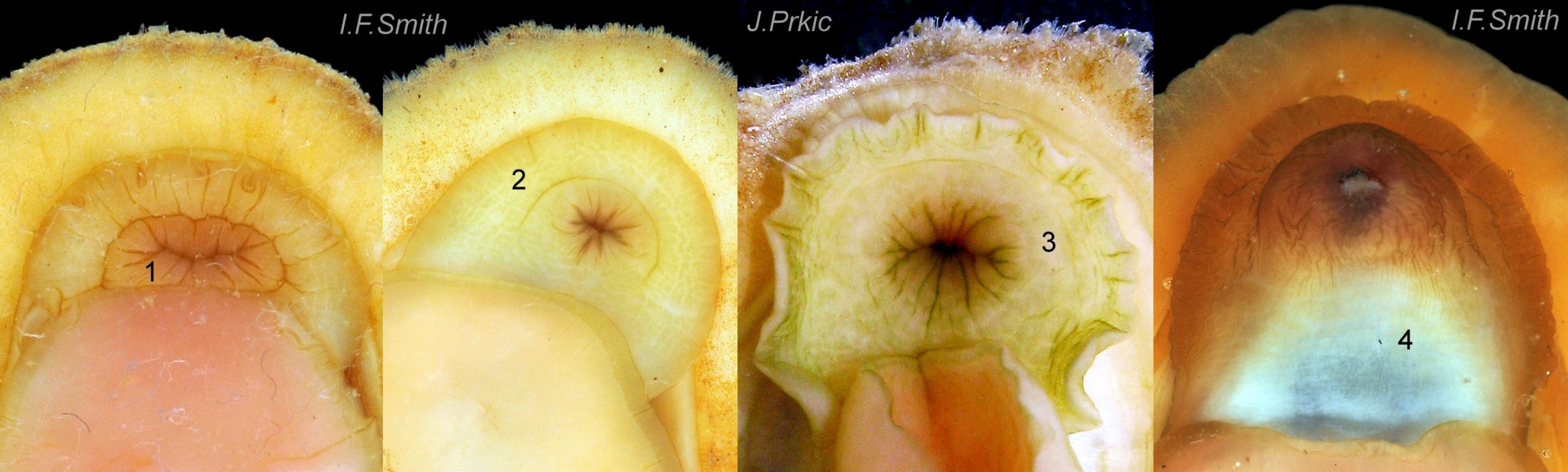
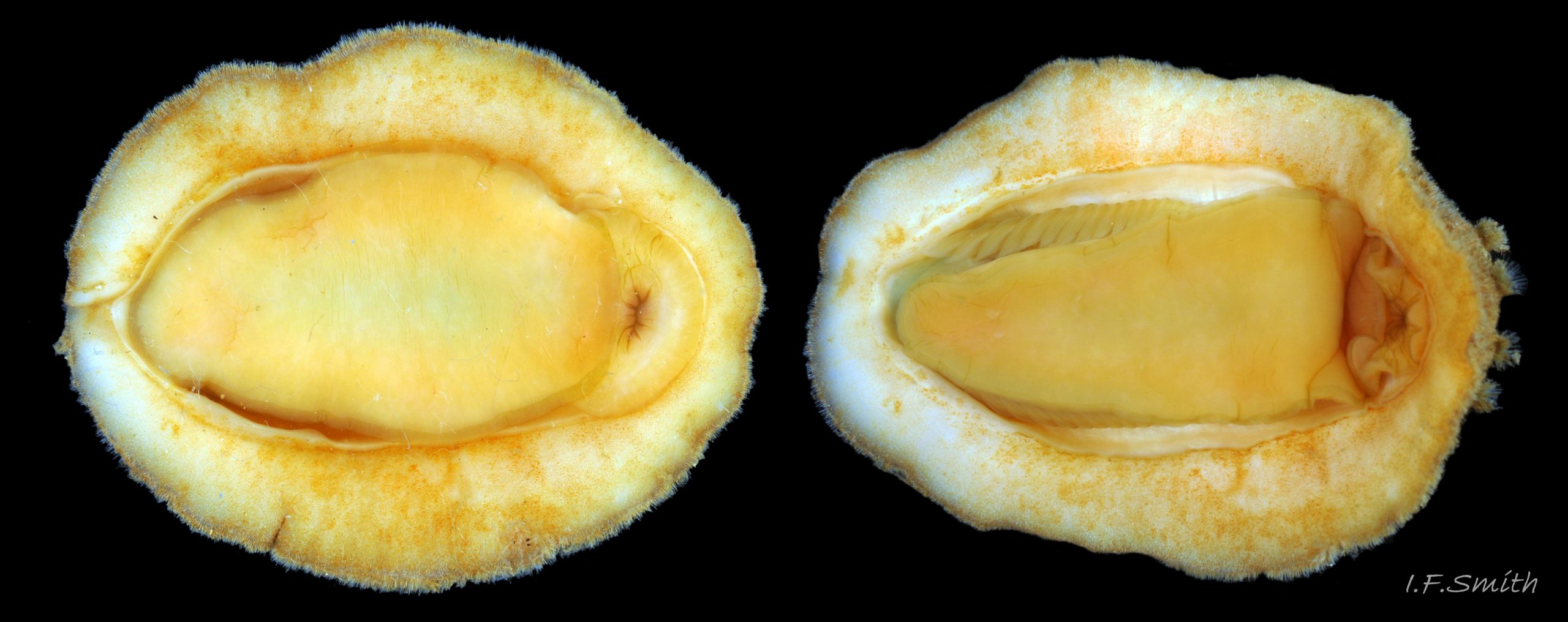
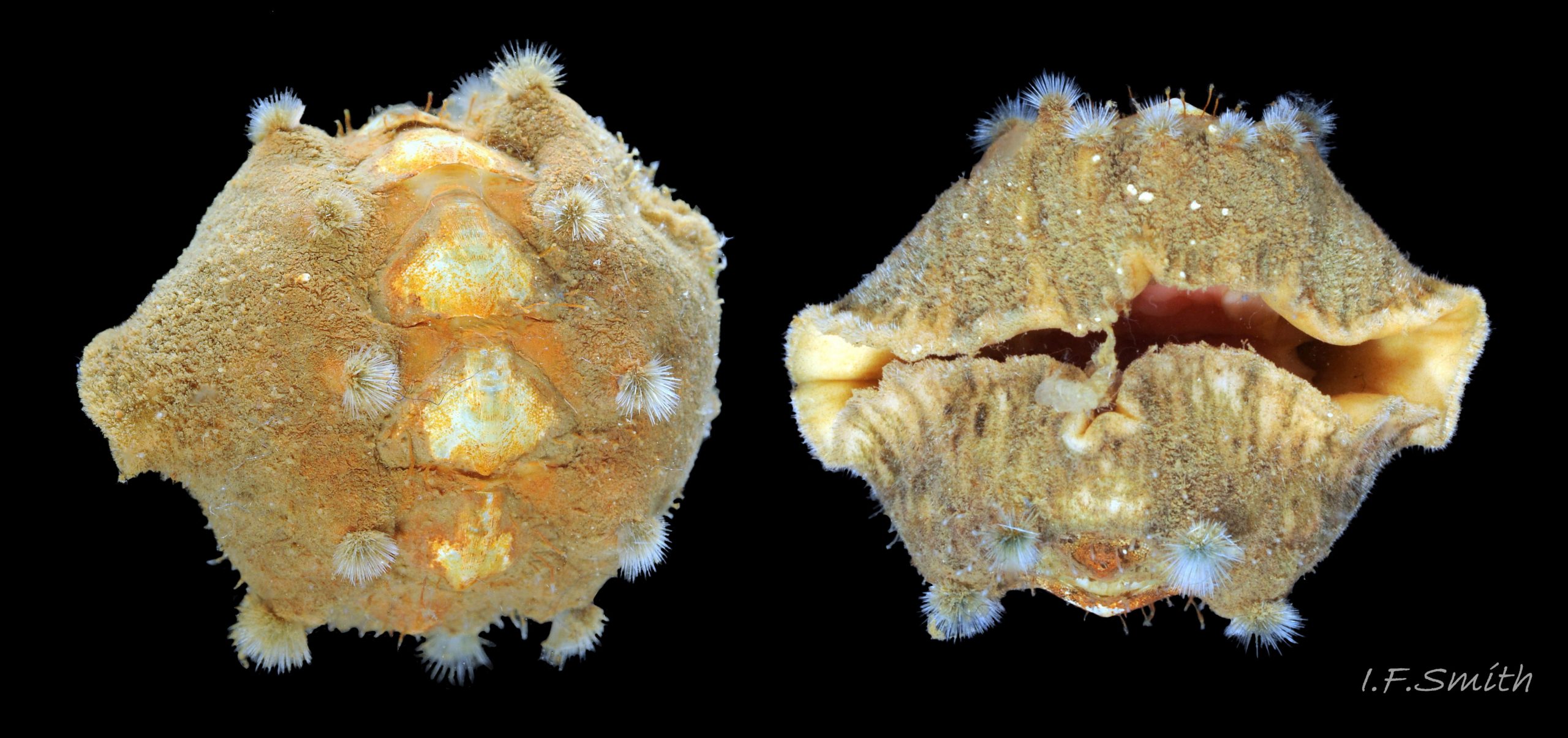
Up to 60mm long; Britain’s largest chiton 01 Acanthochitona fascicularis, 02 Acanthochitona fascicularis . In dorsal view, the elliptical outline varies in width from about 46% of length when extended 03 Acanthochitona fascicularis to 68% when contracted 04 Acanthochitona fascicularis . When removed from the girdle, intermediate valves have a relatively elevated profile with a definite keel 05 Acanthochitona fascicularis .
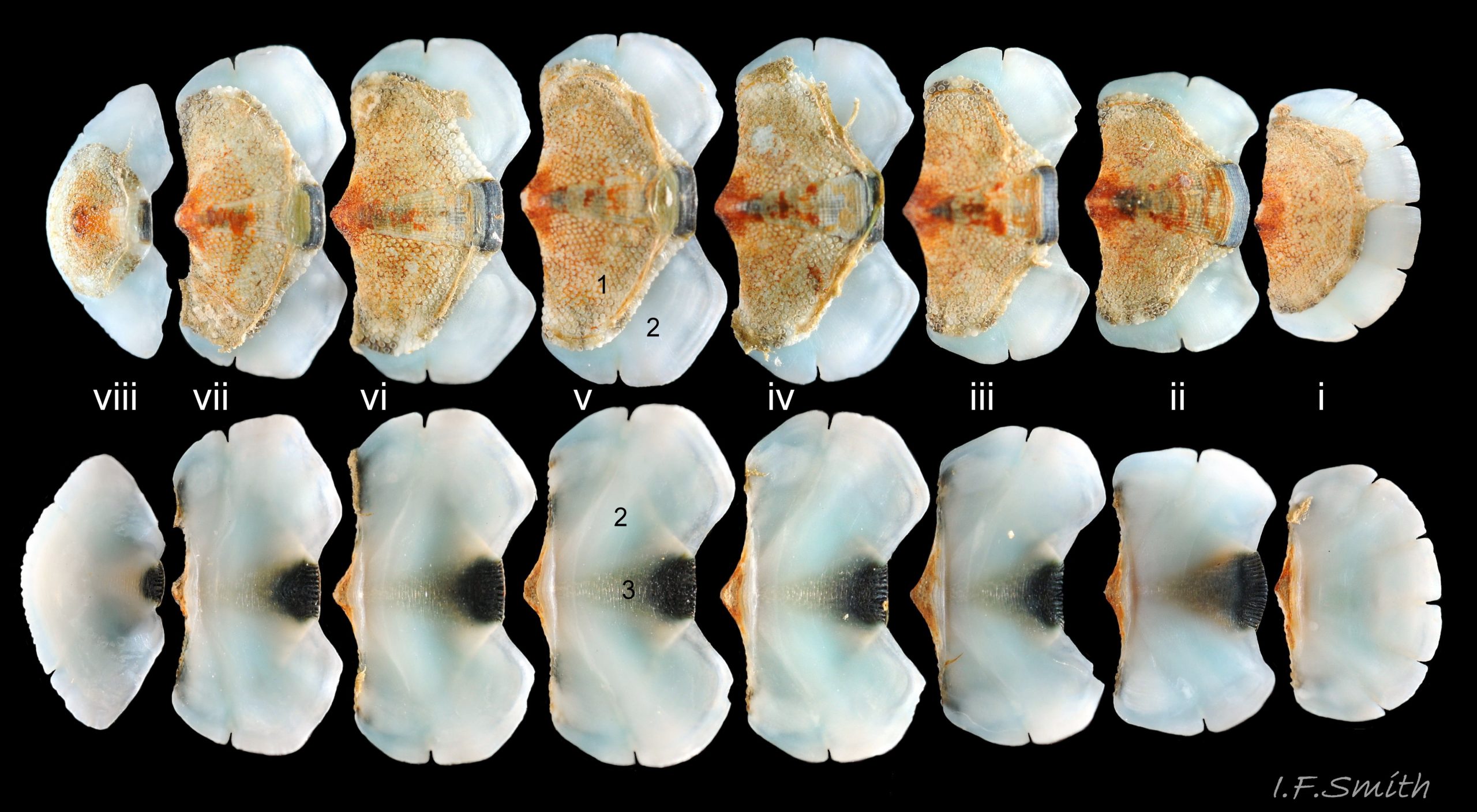
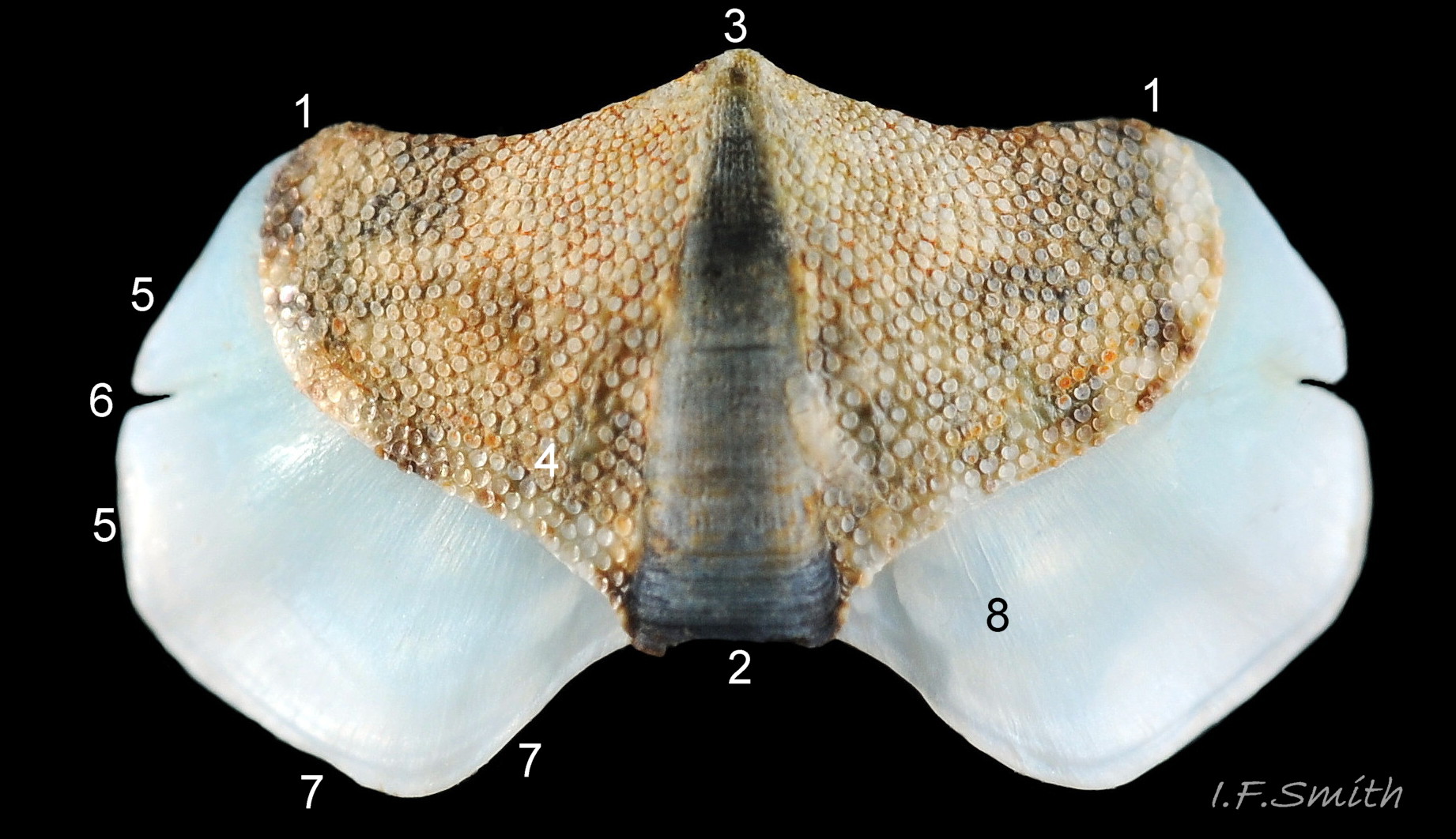
There are eight valves 06 Acanthochitona fascicularis composed of:
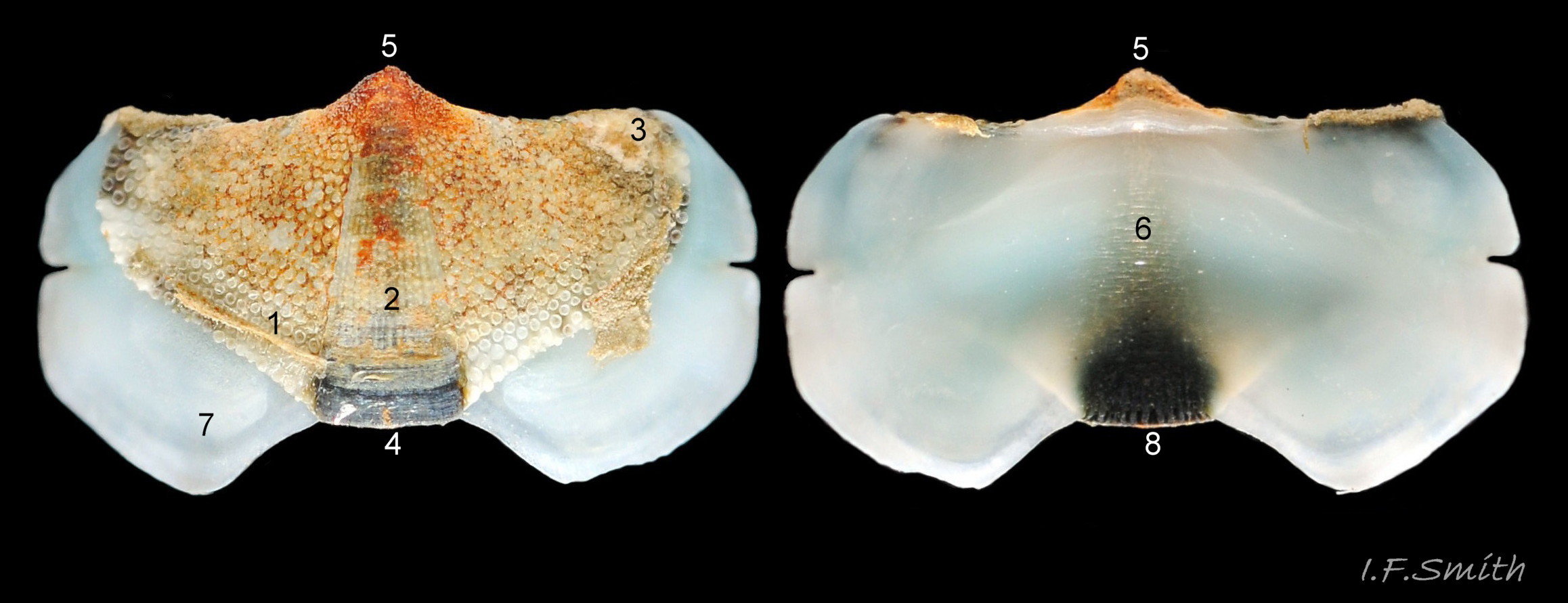
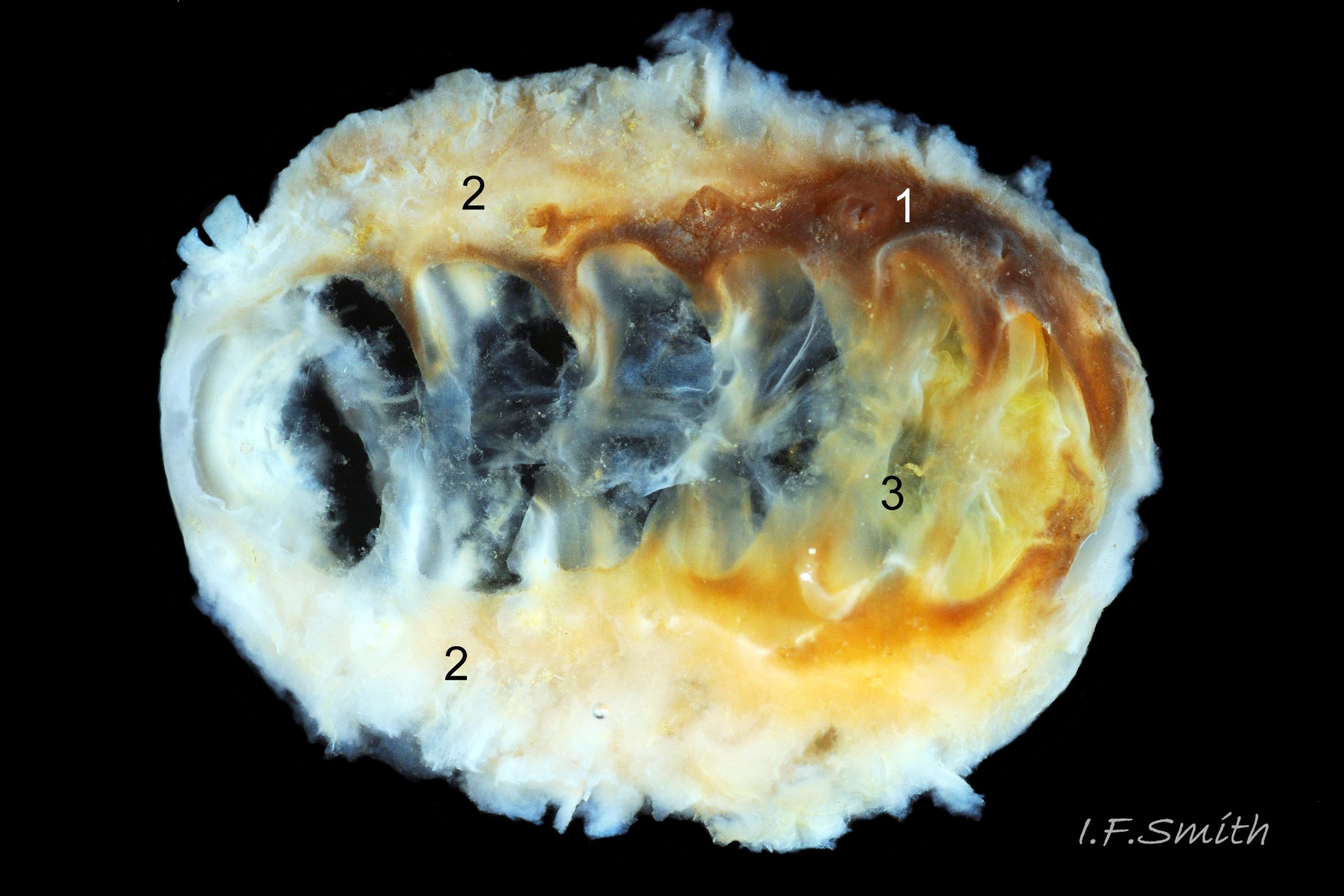
1) The tegmentum, visible on the live animal; a dorsal, sculptured, coloured layer of organic chitinous material and inorganic aragonite 07 Acanthochitona fascicularis & 05 Acanthochitona fascicularis permeated by canals.
2) The articulamentum, concealed on the live animal; a ventral layer of white, nacreous aragonite, permeated by canals only in the jugal area 07 Acanthochitona fascicularis & 05 Acanthochitona fascicularis .
3) The properiostracum; a thin, translucent, outermost proteinaceous layer containing much of the colour.
4) The myostracum; a thin discontinuous innermost layer (only visible in section examined under a microscope).
The aragonite valves are substantial, but brittle when not supported by the chiton’s powerful muscles 08 Acanthochitona fascicularis .
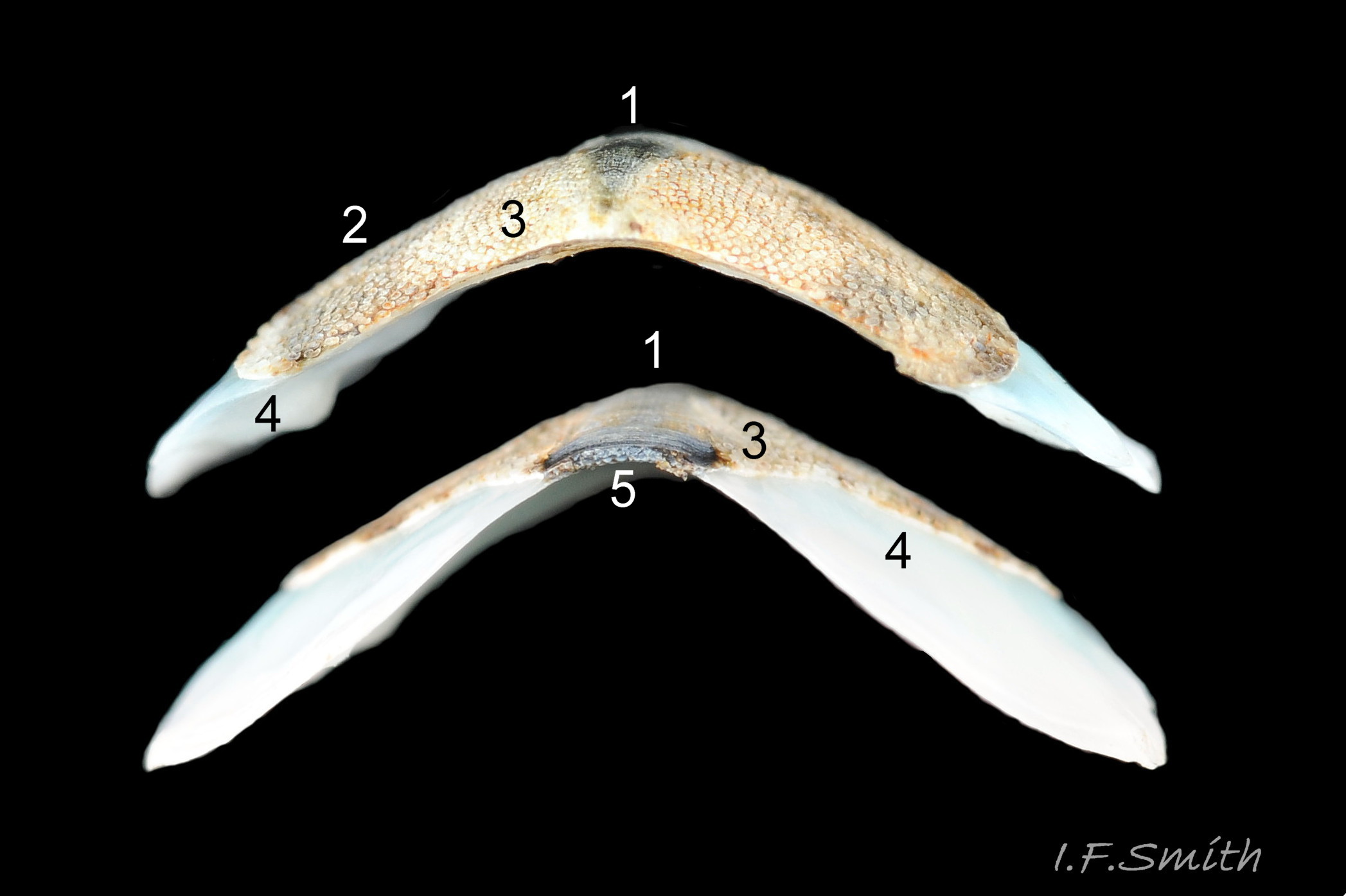
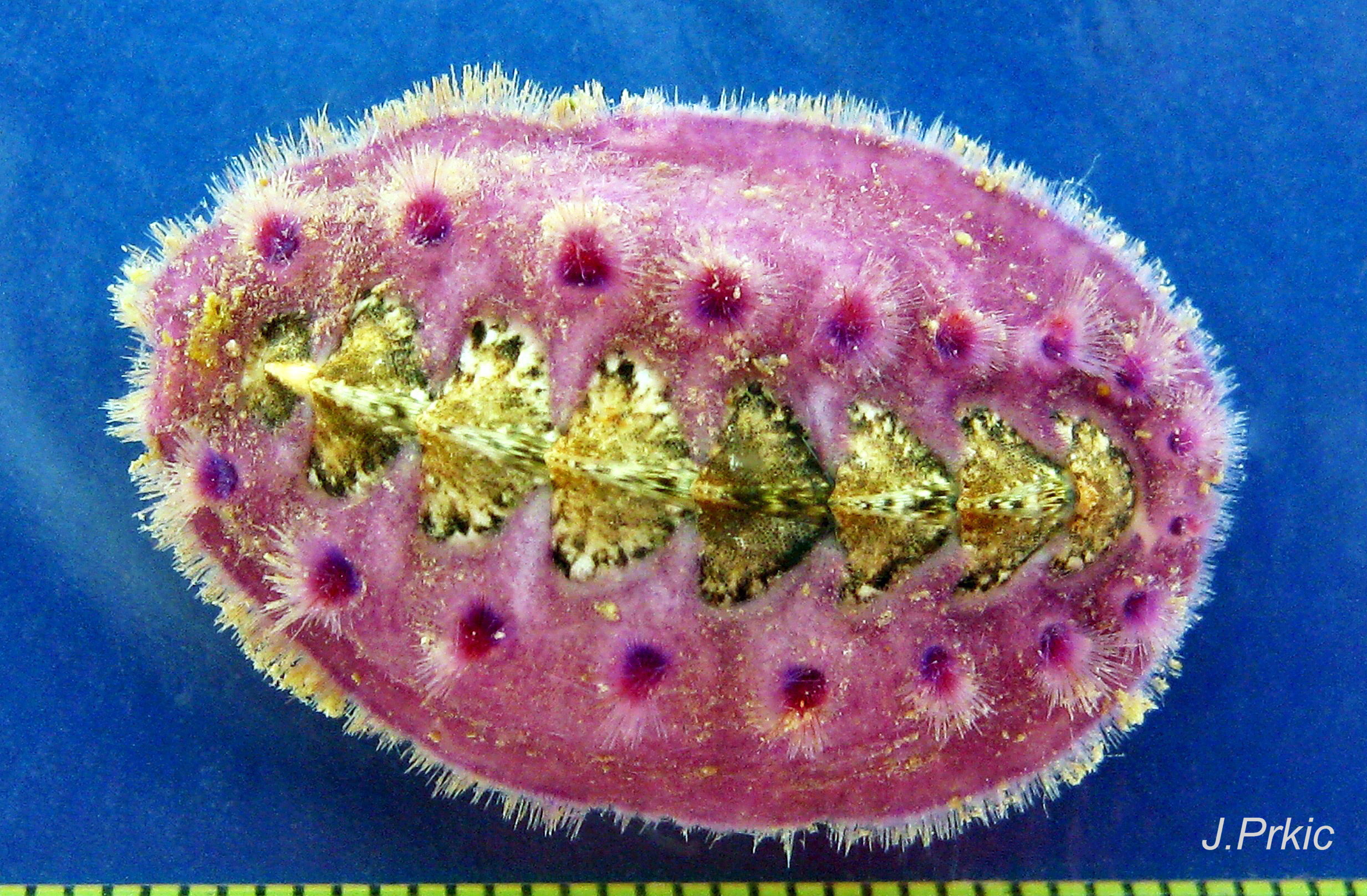
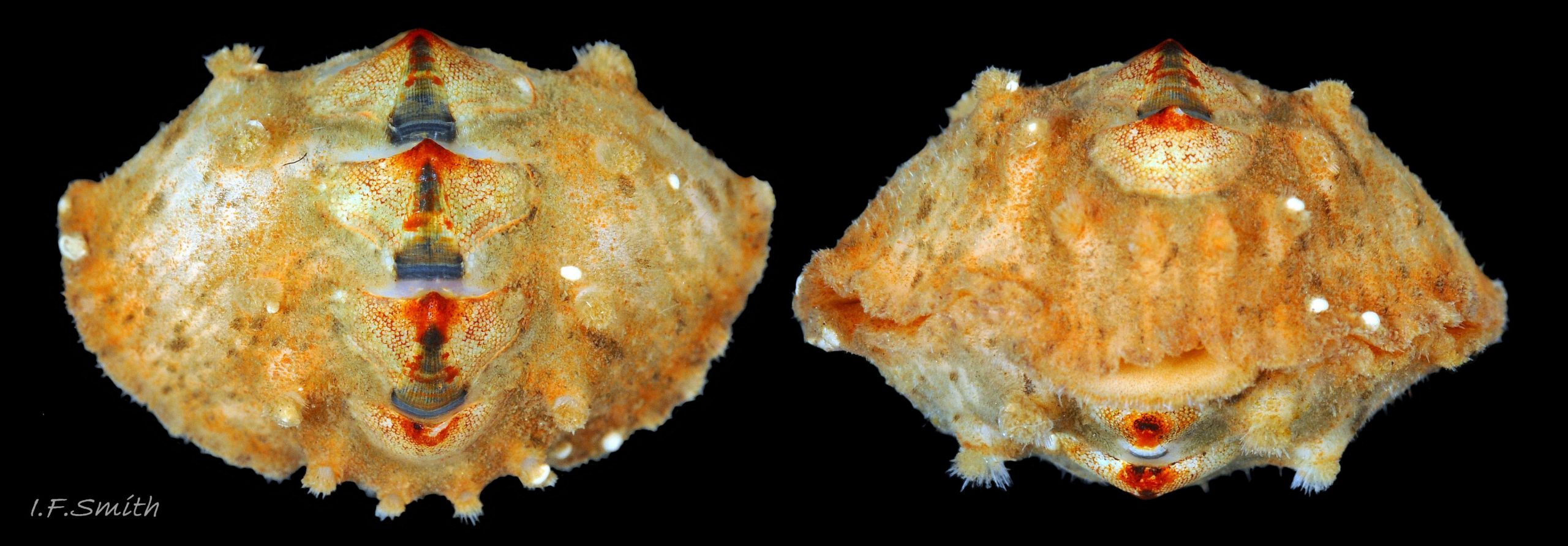
The tegmentum, has crowded, small, circular, evenly distributed granules, except on the narrow triangular jugal area which has longitudinally arranged ridges and grooves 09 Acanthochitona fascicularis . The circular granules have flat tops, often with a raised rim 07 Acanthochitona fascicularis & 10 Acanthochitona fascicularis . The sculpture may be indistinct or missing from old eroded specimens 11 Acanthochitona fascicularis . From above, the tegmentum of intermediate valves (ii to vii) 07 Acanthochitona fascicularis & 10 Acanthochitona fascicularis is approximately broadly triangular with rounded lateral angles, a flattened anterior angle, the jugal sinus, and a protruding posterior beak 12 Acanthochitona fascicularis . Intermediate valves are keeled, with slightly curved side slopes when viewed from the posterior or anterior 05 Acanthochitona fascicularis . Lateral and pleural areas are not differentiated on this species so there is no separating diagonal ridge. Growth lines are obscured by the dense granules 09 Acanthochitona fascicularis , and the whole animal may be coated with detritus 01 Acanthochitona fascicularis . From above, the head valve (i) is approximately semicircular 08 Acanthochitona fascicularis & 13 Acanthochitona fascicularis and the tail valve (viii) is approximately circular 8Af 08 Acanthochitona fascicularis (apparent 2D shape varies with how the 3D valves are positioned). On live specimens, when the white articulamentum is hidden by the girdle, the tail valve looks tiny relative to the other valves, and its mucro barely protrudes 12 Acanthochitona fascicularis . Tegmentum colours (seen through the translucent, coloured properiostracum) are various mottled or streaked combinations of white, cream, yellow, brown, black 03 Acanthochitona fascicularis , orange and red 12 Acanthochitona fascicularis ; the jugal area is often coloured distinctly different from the rest of the valve 09 Acanthochitona fascicularis . Old eroded specimens may have lost their colours apart from environmental staining and the white of the, now exposed, inner layers of the shell 01 Acanthochitona fascicularis .
Canals permeate the tegmentum and terminate on its dorsal surface in the flat-topped granules. Each granule has a single megalaesthete (large pore with underlying canal) accompanied by 0 to 5 micraesthetes (Schmidt-Petersen et al. 2015; not discernible in images in this account) . In the jugal area, the aesthetes are closely arranged on the longitudinal ribs 07 Acanthochitona fascicularis .
The articulamentum is thinner, but about twice as extensive as the tegmentum, protruding from below it into large lateral insertion plates 10 Acanthochitona fascicularis that, when live, are embedded in the wide girdle . They have a single notch separating two plates on each side, except valve i (head valve) with a total of five notches separating six plates 13 Acanthochitona fascicularis . On valves ii to viii, the insertion plates merge anteriorly with very large protruding apophyses 10 Acanthochitona fascicularis that are embedded in tissue under the adjacent valve when live. The articulamentum forms the ventral surface of the valves which is nacreous white, frequently with black on the ventral jugal tract 07 Acanthochitona fascicularis . Sculpture on the articulamentum is confined to faint radial ridges on the apophyses 10 Acanthochitona fascicularis (only visible in certain lights) and protrusion of the jugal ridges at the jugal sinus 07 Acanthochitona fascicularis . Canals penetrate the surface of the articulamentum only in the ventral jugal tract where they are arranged in, often visible, transverse grooves 07 Acanthochitona fascicularis .
Body Description
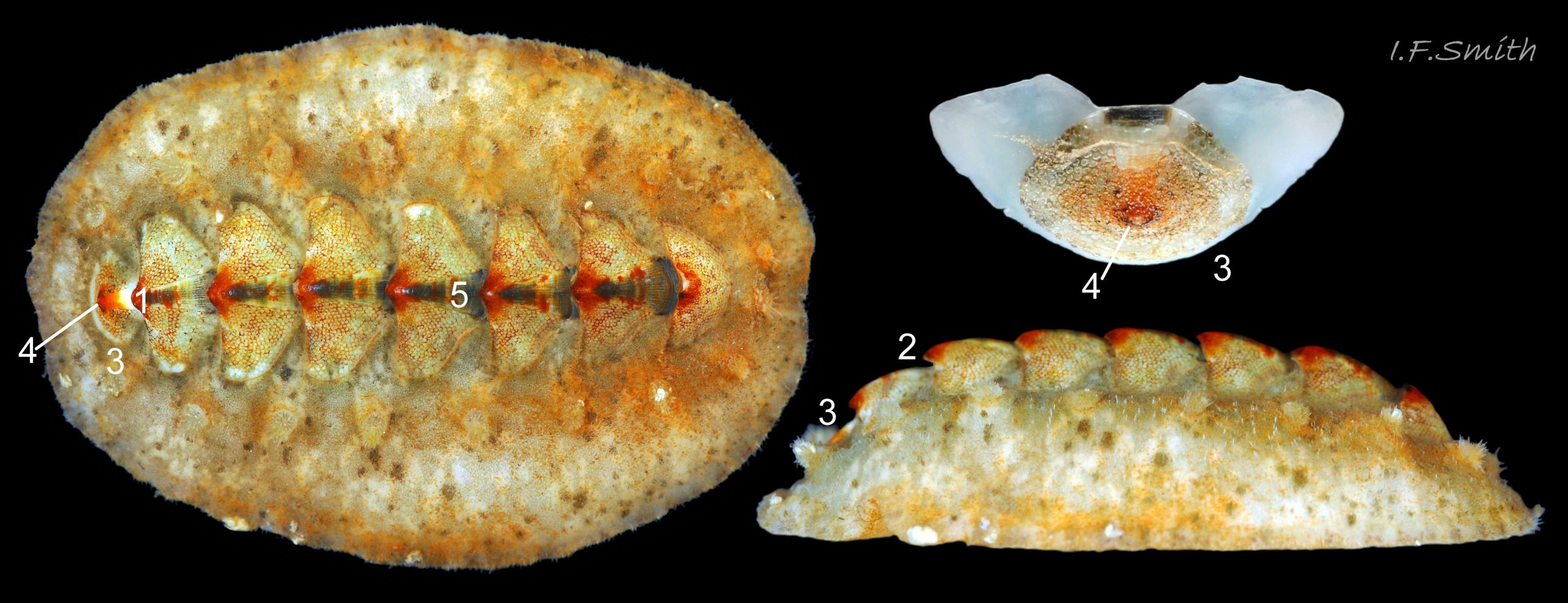
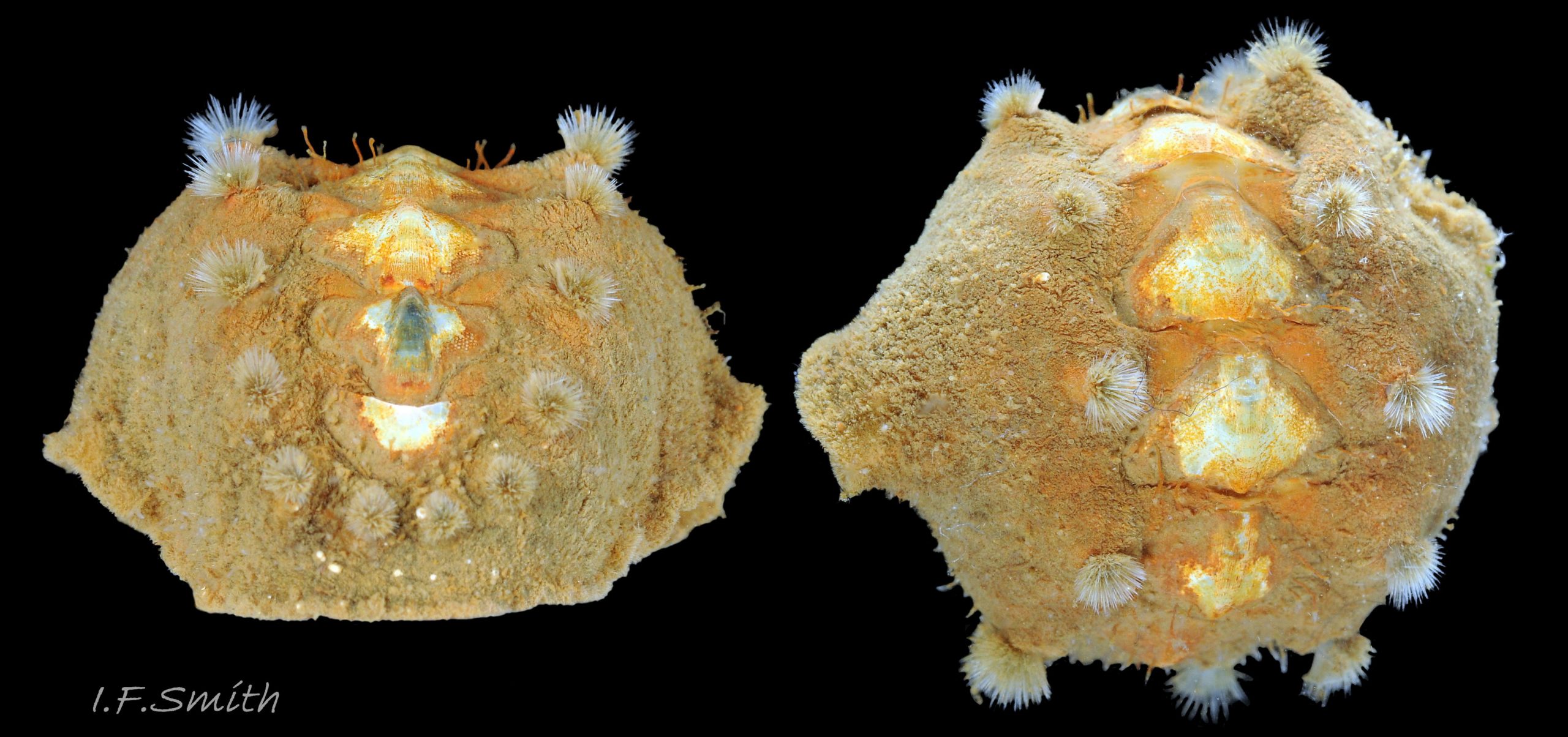
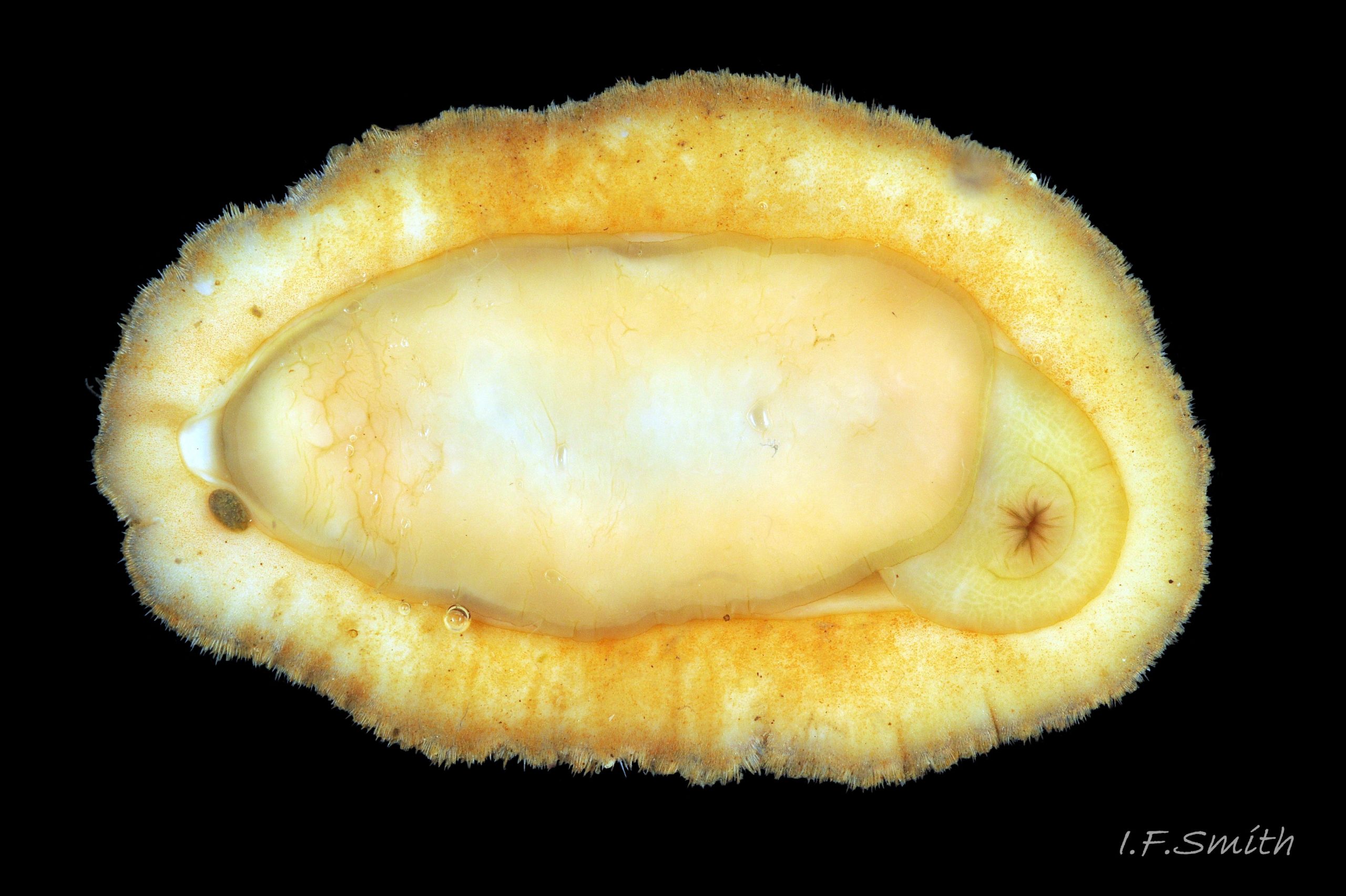
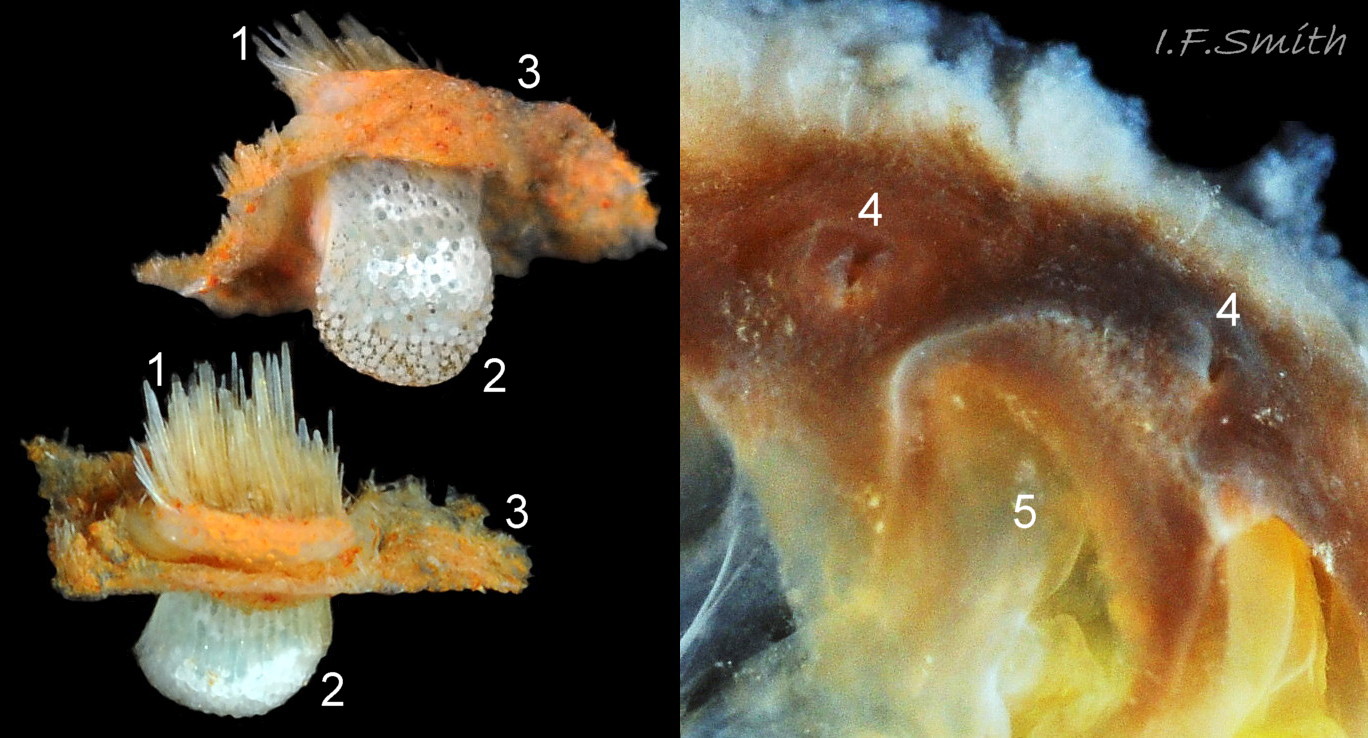
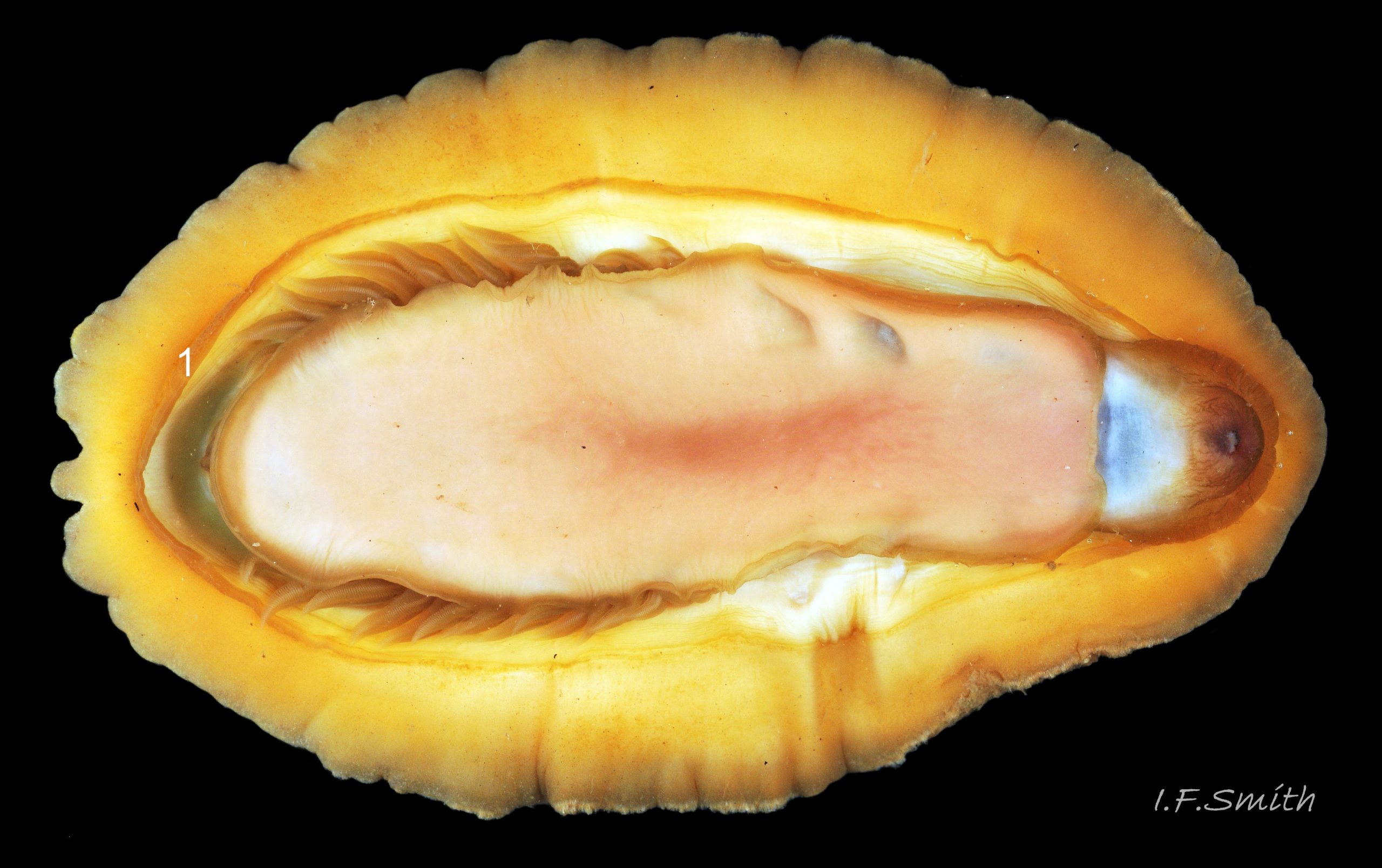
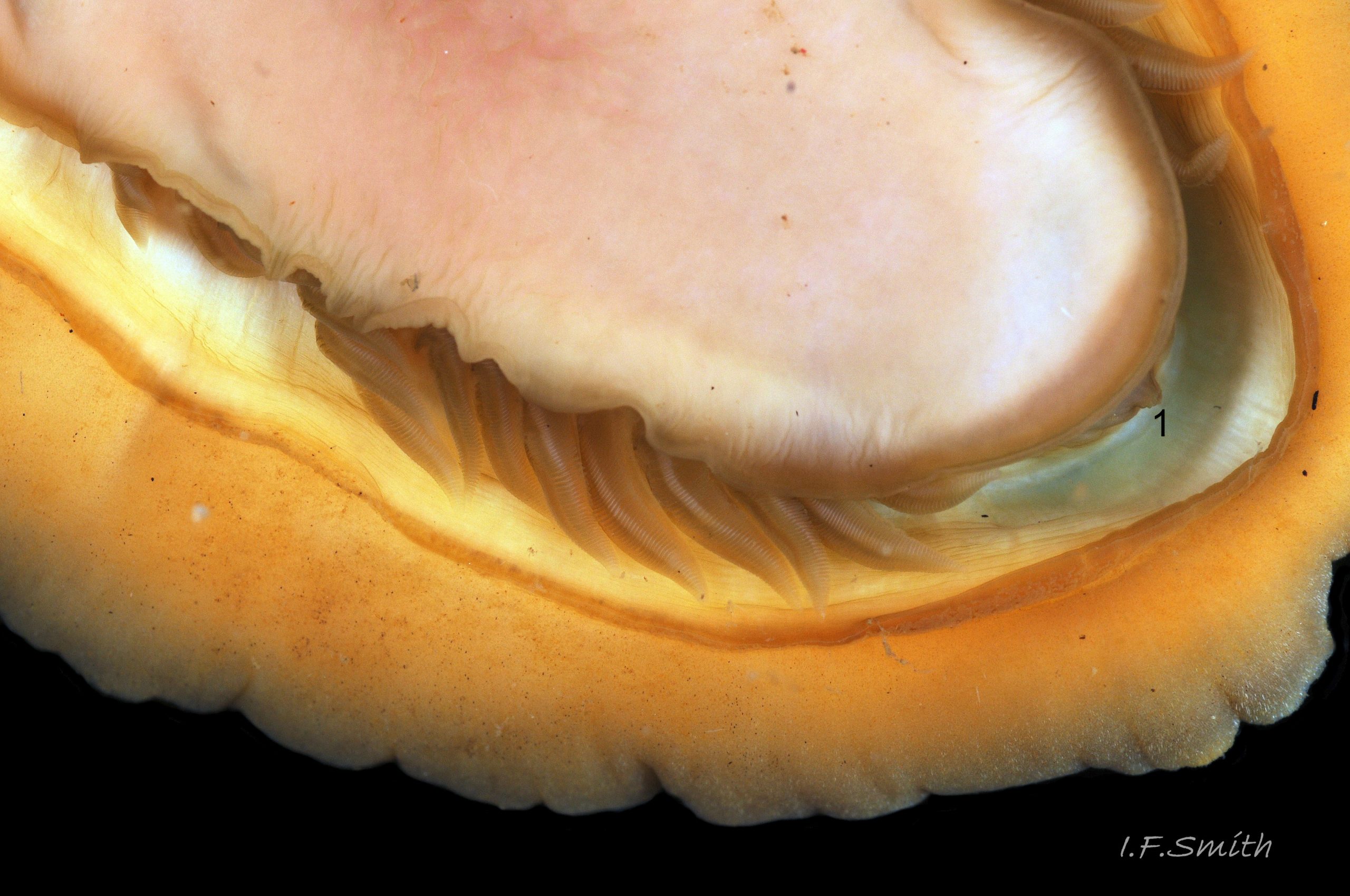
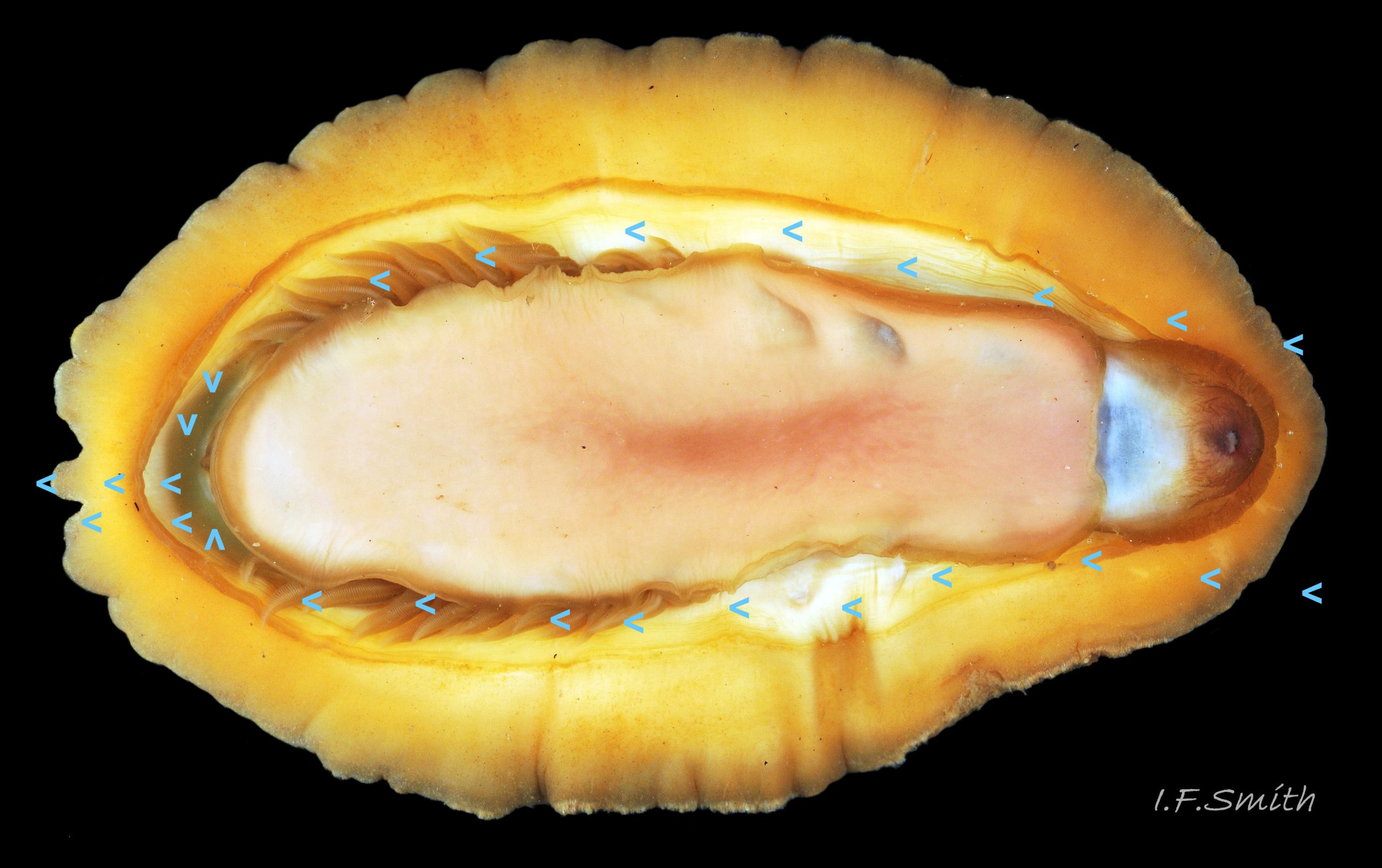
Immediately under the valves, the mantle is a tough, thin, translucent epidermis, but it is greatly thickened where it is reflected around the periphery of the animal to form a very wide, fleshy girdle into which the insertion plates of the valves are firmly embedded 14 Acanthochitona fascicularis , 15 Acanthochitona fascicularis & 16 Acanthochitona fascicularis . The only part of the valves visible on the live animal is the coloured tegmentum. Both absolutely, and relatively to the tegmentum, the girdle on A. fascicularis is the largest of all British chitons 17 Acanthochitona fascicularis . The visible intermediate valves occupy only 30 to 40% of the chiton’s width; the girdle on a single side is greater than 18 Acanthochitona fascicularis , or equal to 19 Acanthochitona fascicularis , the width of the valve. The girdle extends between the visible valves. When the chiton is at rest adhering to the substrate, the extensions from each side almost meet and are only separated by the narrow jugal area measuring about 5% of the animal’s width 18 Acanthochitona fascicularis & 19 Acanthochitona fascicularis ; and they may be seen to meet without a gap when the chiton curls 20 Acanthochitona fascicularis . The perinotum (dorsal surface of the girdle) is covered by a tough cuticle bearing abundant 0.5 mm long, erect, translucent colourless and/or black spicules 21 Acanthochitona fascicularis & 22 Acanthochitona fascicularis and smaller, denser, usually brownish, spicules, length 0.01- 0.015 mm, that form a felt-like cover. Large spicules protrude as a fringe from the rim of girdle 23 Acanthochitona fascicularis ; their appearance varies with the amount of wear experienced 24 Acanthochitona fascicularis and the amount of detritus 01 Acanthochitona fascicularis micro-fibres and epibiota coating the animal . The perinotum also has, adjacent to the anterior edge of each intermediate valve (ii to viii), a sutural tuft of 60 to 120, thin (13µm to 24µm diameter) , straight, needle-like, translucent spicules up to about 2mm long 03 Acanthochitona fascicularis that may be colourless or tinted yellow, brown, pink, magenta or green for part or all of their length 25 Acanthochitona fascicularis . The tail valve (viii) has two tufts at its posterior, and the head valve (i) has four tufts at its anterior, making a total of eighteen tufts 03 Acanthochitona fascicularis . The tufts arise from distinct pockets in the girdle 26 Acanthochitona fascicularis . The hyponotum (ventral surface of the girdle) is also covered by a tough cuticle, but the scale-like spicules coating it are smaller than the spicules on the perinotum 24 Acanthochitona fascicularis . It is often stained brownish from detritus or mineral deposits. The ground colour of the girdle, after any debris is brushed off, can be yellow 02 Acanthochitona fascicularis , ochre 03 Acanthochitona fascicularis , magenta 28 Acanthochitona fascicularis or pink. If the surface cuticle is eroded, the white flesh of the girdle is exposed 27 Acanthochitona fascicularis ,
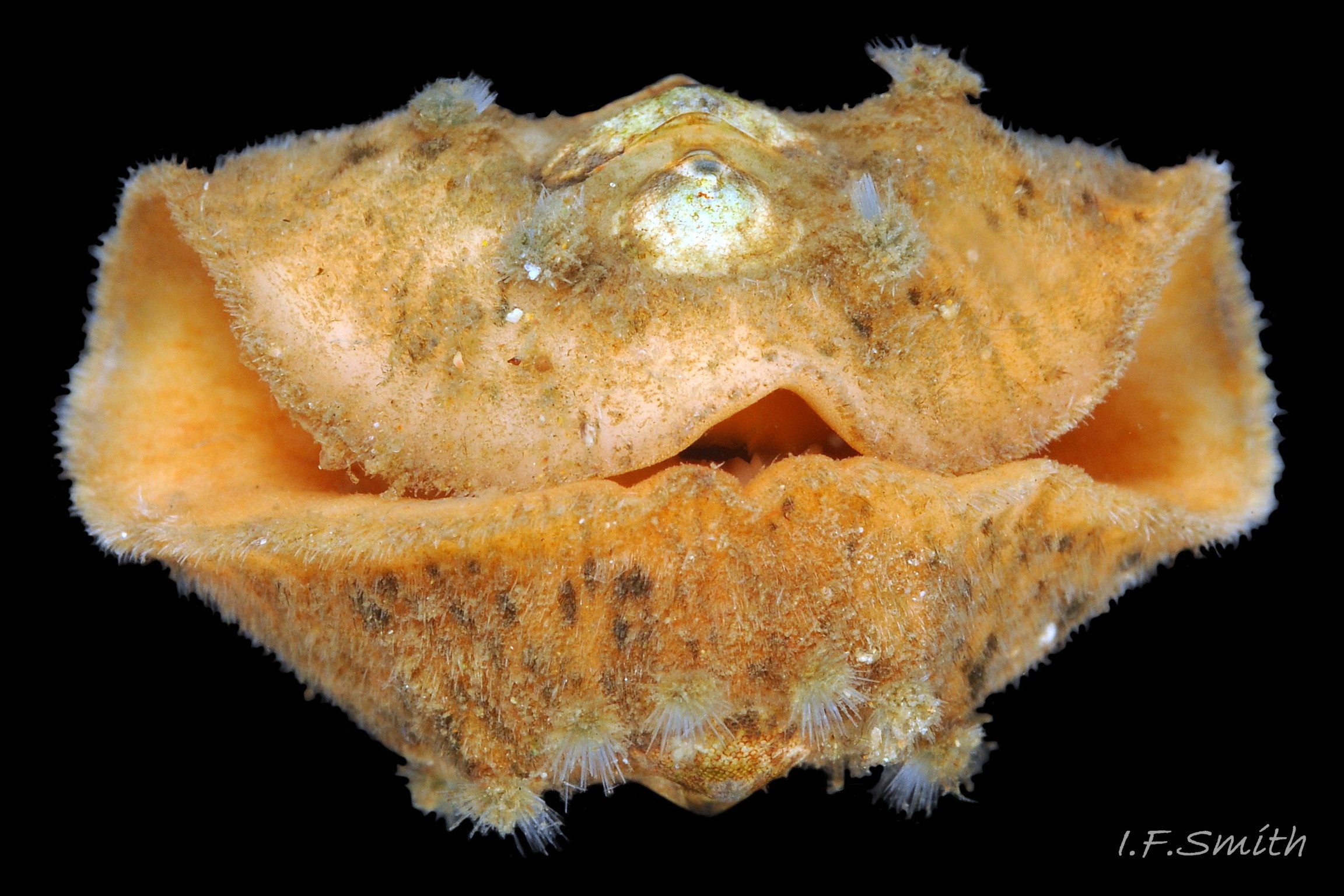
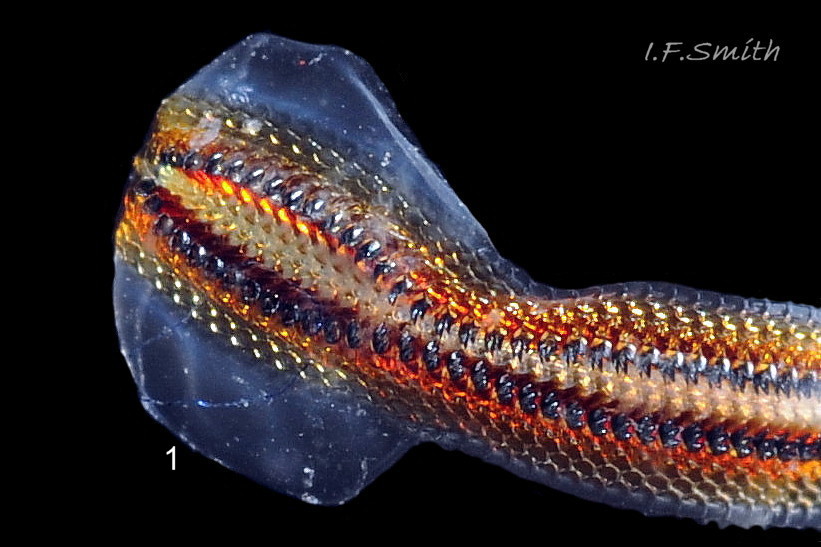
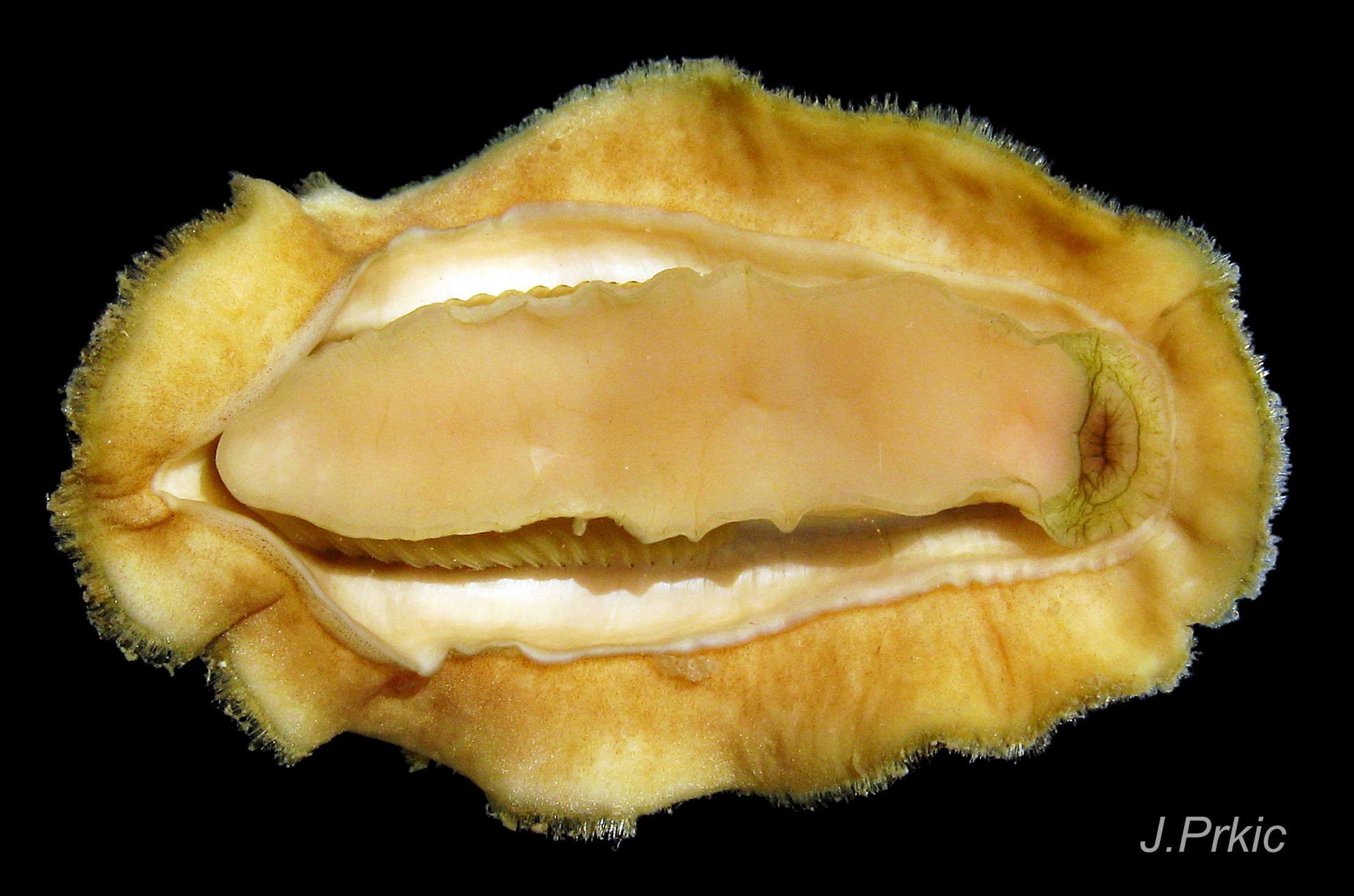
The head and foot never protrude into view naturally on a live animal attached to the substrate, and can only be seen if the animal is detached, or attached to glass 24 Acanthochitona fascicularis . The head lacks eyes and sensory tentacles. It consists of a large transverse slit-mouth with wrinkled lips, surrounded by a large hood 29 Acanthochitona fascicularis . It is usually yellowish, sometimes orange or pinkish, and may be stained green by the algae it feeds on. The ventral surface of the head, usually concealed against the foot, is whitish. As on chitons generally, A. fascicularis has a long (c.33% body length), broad radula 30 Acanthochitona fascicularis with extremely strong, hard teeth of chitin mineralized with magnetite (so iron rich that it adheres to a magnet) in rows of seventeen. The teeth are pale when initiated at the rear of the radular sac 31 Acanthochitona fascicularis . They harden and darken to bright rust and black as they are moved forwards along the radula and incrementally mineralized by the walls of the sac 32 Acanthochitona fascicularis . In each row a pair of tricuspid, major-lateral teeth, the largest and darkest, are the principal scrapers, and a pair of paddle-like major (third) uncinal teeth 32 Acanthochitona fascicularis sweep up loosened food particles. The anterior of the radula is flanked by a hyaline shield 30 Acanthochitona fascicularis and supported by an odontophore when grazing rock surfaces.
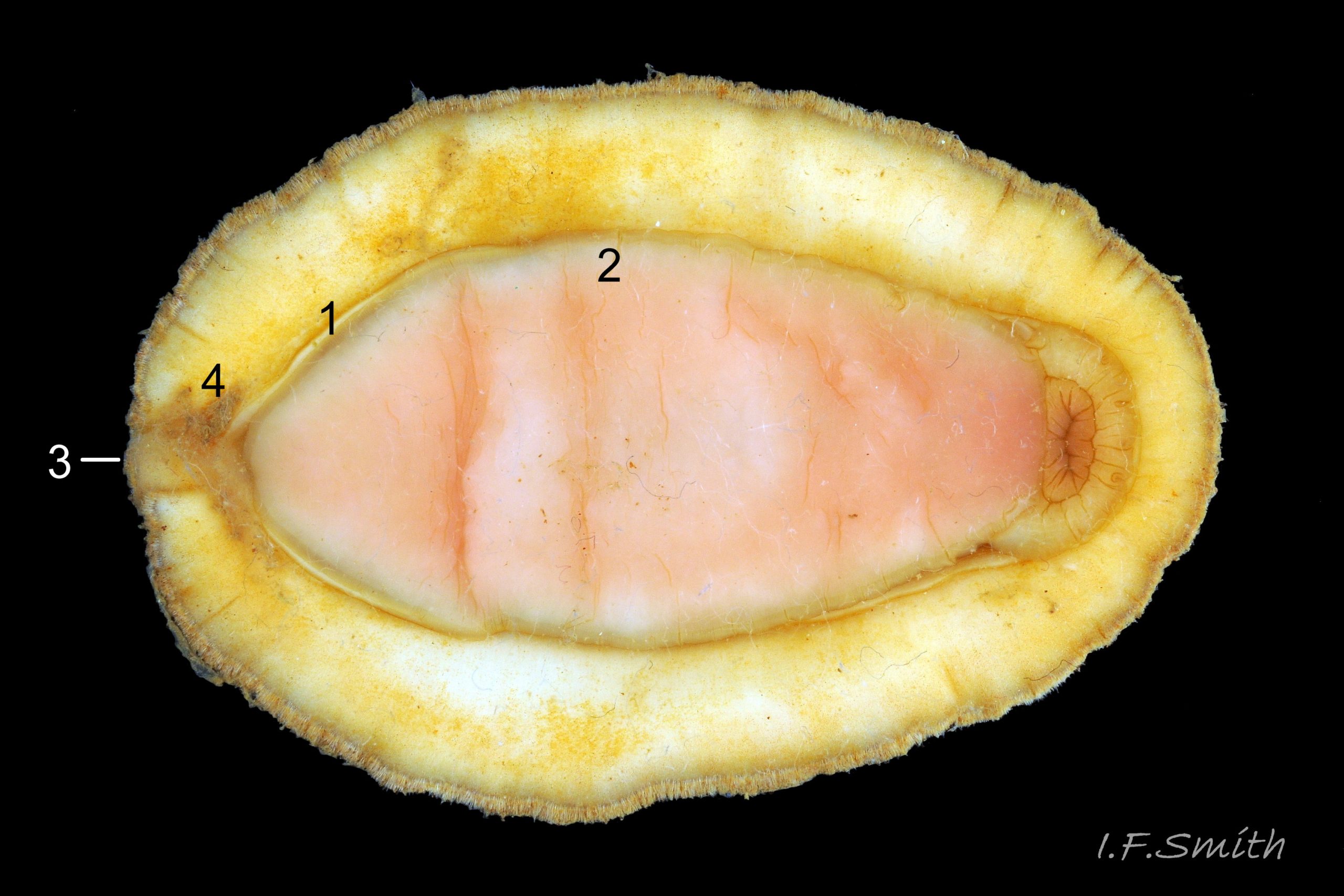
The foot is an elongate ellipse with no medial dividing line. The sole can be yellowish white 24 Acanthochitona fascicularis , salmon pink 33 Acanthochitona fascicularis , or various shades of yellow or buff 34 Acanthochitona fascicularis . Colour saturation increases when the foot contracts 35 Acanthochitona fascicularis .
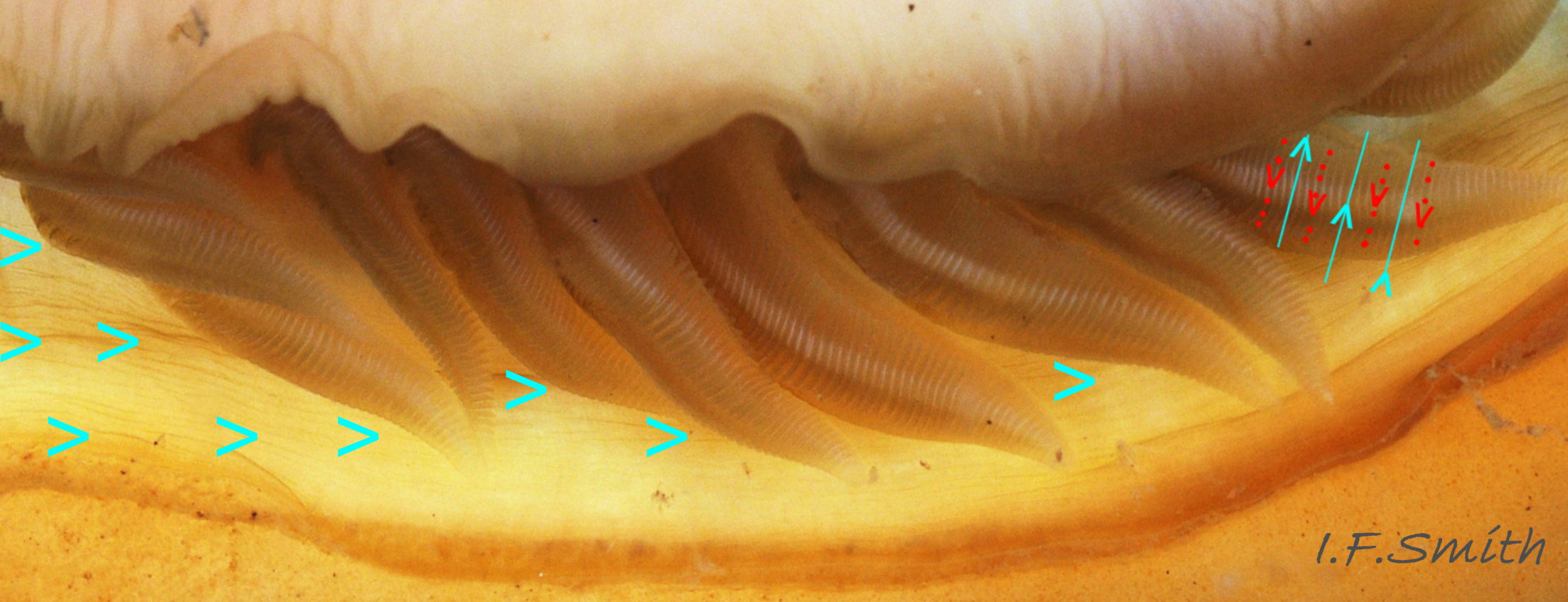
A narrow mantle-cavity/groove runs around the whole animal. It contains about fifteen gills on each side in the posterior two thirds only (merobranch arrangement) 36 Acanthochitona fascicularis & 35 Acanthochitona fascicularis . Each gill has an axis with many lamellae on either side 37 Acanthochitona fascicularis . This resembles the usual molluscan ctenidium, but it may be an independently evolved feature on chitons. Between the mantle cavity and the hyponotum, the mantle-fold 36 Acanthochitona fascicularis may be unobtrusive but when it and the foot are expanded they convert the mantle cavity from a groove to a closed respiratory tube that conceals the gills 33 Acanthochitona fascicularis . The anus, positioned on a papilla 38Af 38 Acanthochitona fascicularis , opens into the posterior of the mantle-cavity. A gonopore and a nephridiopore open on each side into the posterior quarter of the mantle cavity. There is no penis as fertilization is external.
Key identification features
Acanthochitona fascicularis
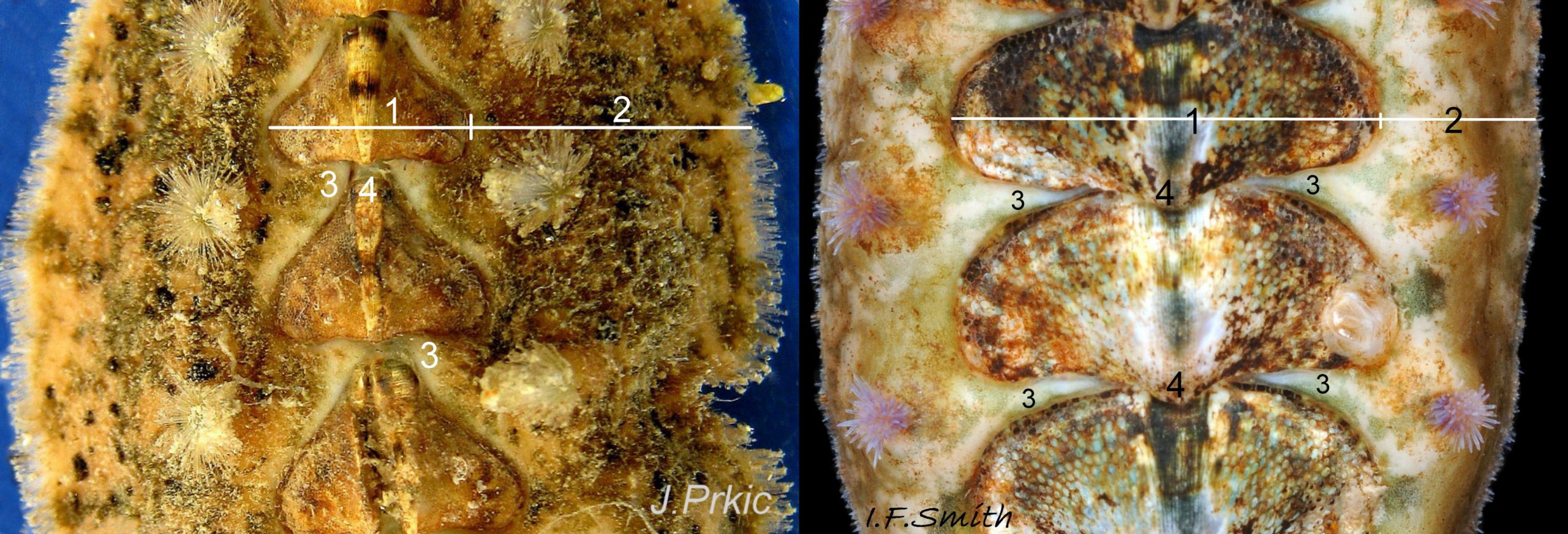
1: Intermediate valve narrower than, or about same width as, girdle to one side of it 45 Acanthochitona fascicularis .
2: Girdle extensions between valves from each side almost meet; gap about 5% of animal’s width 45 Acanthochitona fascicularis .
3: Eighteen tufts each have 60 to 120, thin (13µm to 24µm diameter) , straight, needle-like, translucent spicules up to about 2mm long (Schmidt-Petersen et al., 2015 ) 46 Acanthochitona fascicularis . Spicule colours vary and are not diagnostic 25 Acanthochitona fascicularis .
4: Apart from narrow triangular jugal area, the exposed dorsal surface of valves has crowded, evenly distributed, uniformly small, circular, flat topped, granules 47 Acanthochitona fascicularis .
Similar species
Acanthochitona crinita (Pennant, 1777).
1: Intermediate valve distinctly wider than the girdle to one side of it 45 Acanthochitona fascicularis .
2: Girdle extensions between valves from each side do not almost meet; gap about 20% of animal’s width 45 Acanthochitona fascicularis .
3: Eighteen tufts each have 25 to 80 substantial (40µm to 45 µm diameter), slightly curved, translucent spicules (Schmidt-Petersen et al., 2015 ) 46 Acanthochitona fascicularis . Spicule colours vary and are not diagnostic.
4: Apart from narrow triangular jugal area, the exposed dorsal surface of valves has uncrowded, unevenly distributed, large and small, round/oval and tear shape, flat topped, granules 47 Acanthochitona fascicularis .
Mediterranean Acanthochitona
The tufted species A. oblonga, Leloupe, 1981 and A. pilosa, Schmidt-Petersen, Schwabe & Haszprunar, 2015 occur in the Mediterranean. See www.researchgate.net/publication/281628035_Acanthochitona…
Habits and ecology
A. fascicularis lives on sheltered shores under stones that are partly embedded in water-retentive muddy sand on the lower shore 39 Acanthochitona fascicularis , also under rocks in pools up to MTL and sublittorally to about 50 metres. Eulittoral specimens and, in Croatia, those from shallow water of less than 2m, are often larger than those from deeper water. The shell articulates to conform closely to uneven rock surfaces, and the large muscular foot and flat hyponotum grip the surface firmly 24 Acanthochitona fascicularis . It travels by monotaxic, retrograde compression waves of the sole. When alarmed, it can increase its grip suctorially by raising part of the hyponotum to form a partial vacuum, and if displaced from the substrate, it rolls into a defensive ball held firmly by strong muscles 40 Acanthochitona fascicularis & 41 Acanthochitona fascicularis . Cilia on the gills and mantle create an inhalent respiratory water-current entering the pallial cavity wherever the girdle is raised slightly at the anterior 42 Acanthochitona fascicularis . The current passes through the gills which have a counter current of blood within 37 Acanthochitona fascicularis and then along the mantle cavity as an exhalent current to exit at the posterior where the girdle is slightly raised.
In the of absence of eyes or sensory tentacles on the head, A. fascicularissenses the environment through aesthetes exposed on the surface of its shell. The precise functions of the aesthetes is uncertain, but a focusing lens and receptive retina have been found in aesthetes of some, but not all, chiton species. Other proposed aesthete functions include chemoreception, mechanoreception, replenishment of properiostracum materials, and secretion of protective substances. There are also sensory organs on the girdle, in and around the mouth and at the anterior of the pallial groove. When a stone bearing an A. fascicularis on its underside is turned, the chiton will often promptly move into concealment, probably because of negative phototaxis. In some species of chiton the reaction is reversed in older individuals, perhaps because of erosion, or blockage by detritus, of the aesthetes on the valve surface (Kaas & Belle, 1985).
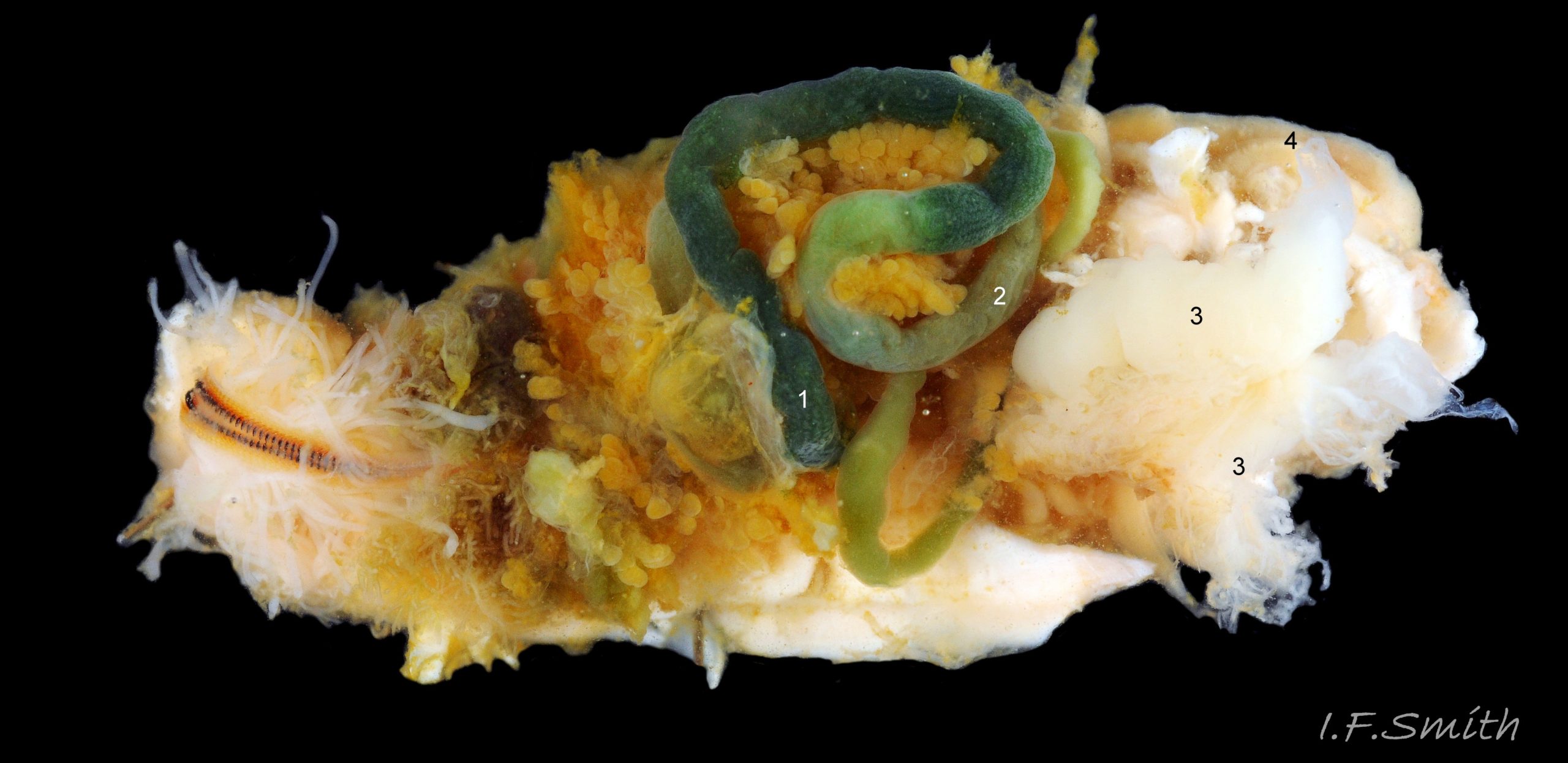
A. fascicularisfeeds by scraping micro algae and associated organisms from the rock surface using its hard radula of chitin mineralized with magnetite 30 Acanthochitona fascicularis . Sometimes the head is stained green by the algae 29 Acanthochitona fascicularis , and the long coiled intestine is usually green 43 Acanthochitona fascicularis . Faeces are compacted in the long intestine into pellets 24 Acanthochitona fascicularis that are carried from the anus, with excreta from lateral nephridiopores, by the exhalent water current of the pallial cavity to be expelled at the posterior from under the slightly raised girdle 33 Acanthochitona fascicularis .
Breeding: Like other chitons, it is dioecious. The large white gonads 44 Acanthochitona fascicularis in the posterior part of the body connect laterally through a left and right gonoduct to the gonopore 43 Acanthochitona fascicularis located level with the suture between valves vi & vii. The exhalent water current in the pallial cavity carries sperm or ova from the gonopores to the posterior and out under the raised girdle . As fertilization is external, synchronised emission of sperm and ova is needed to ensure success; the trigger in some chiton species is moon-phase/state of tides, or it may be initiated by the emission of sperm by one or more males (Kaas & Belle, 1985). Planktonic, trochophore larvae hatch from the ova and metamorphose into small adult-form young without an intervening veliger stage.
Distribution and status
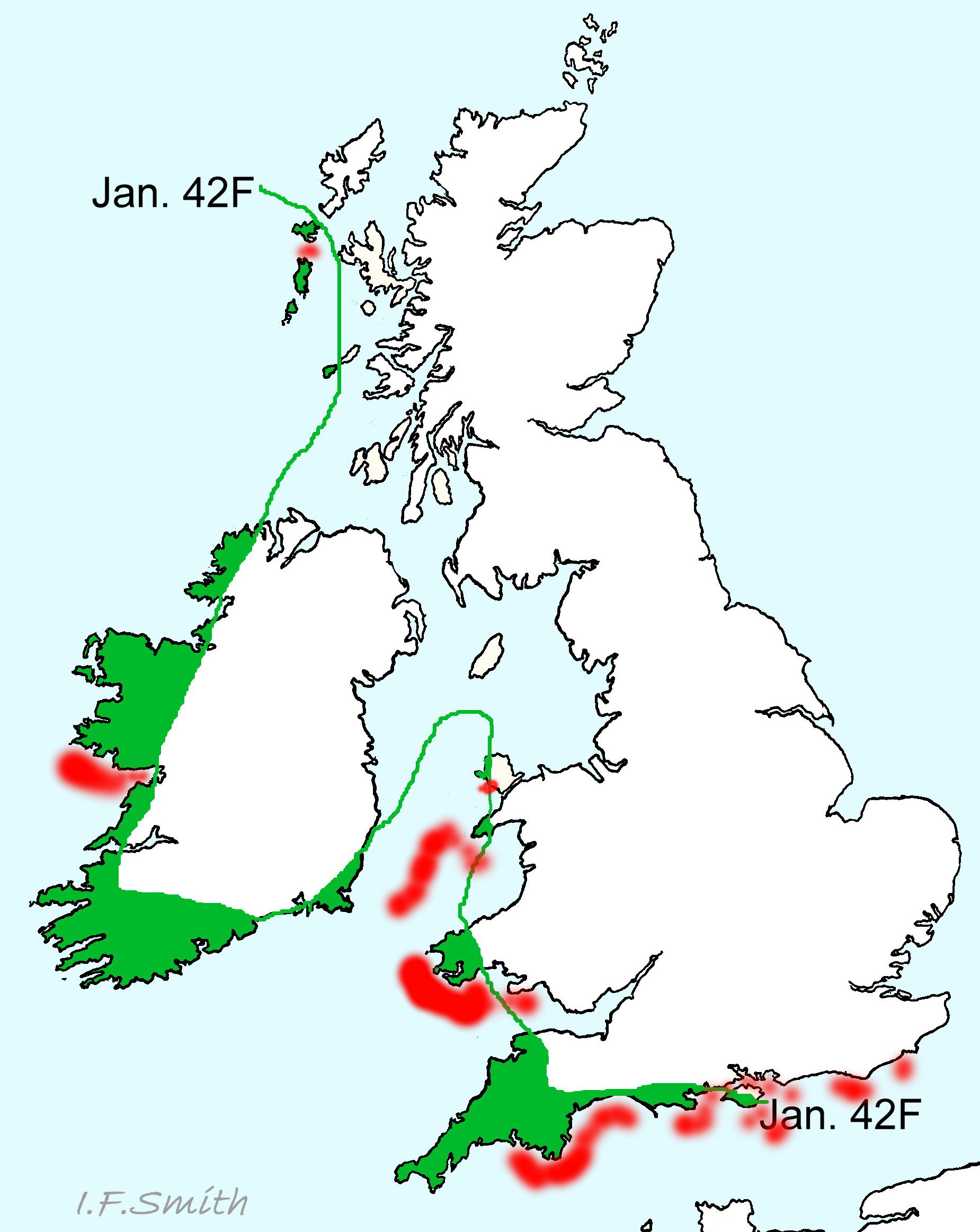
A. fascicularis is a southern species found from the Black Sea, Mediterranean, Canary Islands and Azores, northwards as far as Brittany and the south coast of England; GBIF map www.gbif.org/species/2305886 . It is very common in Croatia, less so in southern England, though fairly numerous locally at favoured sites, and there are records from south Wales, west Anglesey, west Ireland and sublittorally from the southern half of the Irish Sea 48 Acanthochitona fascicularis ; NBN map species.nbnatlas.org/species/NBNSYS0000173555 . A scatter of records further north on GBIF as far the Lofoten Islands in Arctic Norway should be treated with caution.
Acknowledgements
I am most grateful to Steve Trewhella for guiding me to a population of A. fascicularis, and to Jakov Prkić for use of his images and much information. I also thank Neil Ward for use of an image of A. crinita .
References and links
Caution is needed when using published identification aids as most contain inaccuracies. For example, Jones & Baxter (1987) and Hayward and Ryland (1995) have drawings of ‘A. fascicularis‘ showing girdle width and inter-valve extensions that match A. crinita and not A. fascicularis. The details shown incidentally in Schmidt-Petersen et al. (2015) while describing a new species are recommended.
Ávila S, Sigwart J (2013). New records for the shallow-water chiton fauna (Mollusca, Polyplacophora) of the Azores (NE Atlantic). ZooKeys 312: 23–38. zookeys.pensoft.net/articles.php?id=3292
Botelho, A. 2013. Zoologger: mollusc grows hardest teeth in the world New Scientist 3rd October 2013 www.newscientist.com/article/dn24329-zoologger-mollusc-gr…
Carefoot, T. A snail’s odyssey. Learn about chitons, gas exchange. www.asnailsodyssey.com/LEARNABOUT/CHITON/chitGase.php accessed 11th January, 2018.
Dell’Angelo, B. & Smriglio, C. 1999. Chitoni viventi Mediterraneo. Rome, Evolver.
Fernandez, C.Z., Vendrasco, M.J. & Runnegar,B. 2007. Aesthete canal morphology in twelve species of chiton (Polyplacophora). The Veliger 49(2) 51 – 69 www.researchgate.net/publication/246548141_Aesthete_Canal…
Forbes, E. & Hanley S. 1849-53. A history of the British mollusca and their shells. vol. 2 (1853) London, van Voorst. [As Chiton discrepans Brown. F&H misapplied the name Chiton fascicularis Linnaeus to Acanthochitona crinita (Pennant, 1777)]; Free pdf at archive.org/stream/historyofbritish02forbe#page/396/mode/2up Use slide at base of page to select pp.396-397.
Fox, R. 2007. Invertebrate Anatomy On Line. Katharina tunicata lanwebs.lander.edu/faculty/rsfox/invertebrates/katharina….
Hayward, P.J. & Ryland, J.S. 1995. Handbook of the marine fauna of north-west Europe. Oxford, Oxford University Press.
Jardim, J.A. & Simone, L.R.L. 2010. Redescription of Hanleya brachyplax (Polyplacophora, Hanleyidae) from the south-southeastern Brazilian coast. Pap. Avulsos Zool. (São Paulo) 50 no.40
www.scielo.br/scielo.php?pid=S0031-10492010004000001&…
Jeffreys, J.G. 1862-69. British conchology. vol. 3 (1865). London, van Voorst. [Probably as Chiton discrepans Brown; description imprecise. Jeffreys misapplied the name Chiton fascicularis Linnaeus to Acanthochitona crinita (Pennant, 1777)]; Free pdf at archive.org/stream/britishconcholog03jeffr#page/214/mode/2up . Use slide at base of page to select pp.214-215.)
Jones, A.M. & Baxter, J.M. 1987. Molluscs: Caudofoveata, Solenogastres, Polyplacophora and Scaphopoda London, Linnean Society, and Estuarine and Brackish-water Sciences Association.
Kaas, P. & Van Belle, R.A. 1985. Monograph of living chitons Vol 1. Viderup, Denmark. Brill. Preview at
books.google.co.uk/books?id=CKautf2EUPkC&pg=PA7&s…
Matthews, G. 1967. The identification of British chitons. Papers for Students No.9. London, Conchological Society of Great Britain and Ireland.
Naturalis Biodiversity Center www.naturalis.nl/en/
Schmidt-Petersen, J., Schwabe, E., Haszprunar, G. 2015. Acanthochitona pilosa spec. nov., a new species of Acanthochitona Gray, 1821 from the Mediterranean (Mollusca, Polyplacophora). Spixiana 38(1): 11-20. www.researchgate.net/publication/281628035_Acanthochitona…
Schwabe, E. 2010. Illustrated summary of chiton terminology. Spixiana 33(2):171-194.
Free pdf at verlag-pfeil.de/04biol/pdf/spix33_2_02.pdf
Glossary
μm = 0.001 mm
aesthete = (in chitons) one of complex of canals filled with sensory tissue that permeate tegmentum and parts of articulamentum. Occur in bundles of a large megalaesthete accompanied by micraesthetes that open as sensory macropores and micropores on dorsal surface of valves. Some are photoreceptors; other function(s) uncertain, may include chemoreception, mechanoreception, properiostracum replenishment and/or secretion of protective substances.
a.k.a. = also known as.
antemucronal area = area situated to anterior of mucro.
apophysis (pl. apophyses) = natural protruberance within a shell for attachment of muscles. On chitons, anterior extension of articulamentum which underlies preceding valve; on all valves except head valve.
aragonite = orthorhombic crystalline mineral-form of calcium carbonate www.minerals.net/mineral/aragonite.aspx . Less common on land than calcite, but, currently, the more frequent mineral-form in oceans and living mollusc shells.
articulamentum = ventral shell-layer of chiton valves, usually hard, white, porcelaneous aragonite and often differently coloured in central part. (Partially overlain ventrally by inconspicuous myostracum layer.)
buccal mass = anterior of digestive system including an odontophore that supports anterior of radula, and a complex of muscles to operate them and other mouthparts. Often red or pink with myoglobin.
caecum = a blind pouch (cul de sac)
calcite = trigonal crystalline mineral-form of calcium carbonate www.minerals.net/mineral/calcite.aspx . More common on land than aragonite, but, currently, the less frequent mineral-form in oceans and living mollusc shells. More resistant than aragonite to acid rain corrosion; forms outer shell layer of shore-dwelling Littorina species in cool climates. (Corrosion of calcium carbonate faster at cold temperatures).
chemoreception = sensing of chemicals; “smell / taste”.
cilia = (pl.) microscopic linear extensions of membrane that move in rhythmic waves to create locomotion, or move particles and liquids e.g. inhalent water currents. (“cilium” singular). (scanning electron microscope image at 18 Lepidochitona cinerea )
ciliary = (adj.) relating to or involving cilia.
ciliated = (adj.) coated with cilia.
chitin = semitransparent flexible horny protein.
chitinous = (adj.) made of chitin.
coll. = in the collection of (named person or institution) (compare with legit).
ctenidium = comb-like molluscan gill; usually an axis with a row of filaments either side.
ctenidia (pl.) = comb-like molluscan gills; usually an axis with a row of filaments either side.
dioecious = having separate male and female individuals, not hermaphrodite.
direct = (of locomotion waves on foot) waves travel from posterior to anterior.
distal = away from centre of body or point of attachment.
epibiota = organisms living on surface of another organism.
ELWS = extreme low water spring tide (usually near March and September equinoxes).
geotactic (adj.) = of species that moves towards pull of gravity (positively geotactic) or away from it (negatively geotactic). (synonym: gravitaxic).
geotaxis = the positive (or negative) response of a freely moving organism to (or against) gravity.
girdle = (on chiton) peripheral band of thickened, reflexed mantle that encloses articulamentum of valves.
gonopore = opening through which eggs or sperm are released.
holobranch (of chitons) = arrangement of gills in pallial groove that extends full length of foot.
hyponotum = ventral surface of chiton’s girdle.
in litt. = (abbreviation of Latin “in litteris”) in upublished correspondence.
insertion plate = (on most chitons) extension of articulamentum on lateral margin of intermediate valves, anterior margin of head valve and posterior margin of tail valve. Inserts into, and anchors valve to, the girdle muscle block.
intermediate valve = (of chiton) any valve (ii to vii), except head valve (i) and tail valve (viii).
jugal area = (on dorsal surface of a chiton shell) triangular middle section of central area of intermediate valves with apex pointing to posterior; discernible when defined by differences of colour and/or sculpture.
jugal tract = (on ventral surface of a chiton shell) triangular middle section of central part of intermediate valves, with apex pointing to posterior; (defined on A. fascicularis by aesthete pores densely arranged in transverse grooves).
jugum = triangular middle section of central area of intermediate valves. (See jugal area and jugal tract.)
lateral area = (on intermediate valve of chiton) triangular area with its base along lateral edge of valve and its apex near the centre of the posterior edge. a.k.a. lateral triangle.
legit = (abbreviation; leg.) collected/ found by (compare with coll.)
LWS = low water spring tide, two periods of a few days each month when tide falls lowest.
magnetite = mineral of iron oxide, hardest material made by any living organism (Botelho, 2013).
major lateral teeth = (on chiton) largest two teeth in each row of seventeen on chiton radulae; principal scraping blades. Often darkened more than other teeth by high magnetite mineralization.
mantle = sheet of tissue covering visceral mass of molluscs. Secretes shell of shelled species. On chitons, forms pallial cavity/groove and is toughened to form the girdle surrounding the shell valves.
megalaesthete = (see aesthete).
merobranch = (of chitons) gills in pallial groove only in posterior two-thirds of animal.
micraesthete = see aesthete.
monotaxic = (of locomotion waves on foot) single series of waves across complete width of foot.
MTL = mid tide level.
mucro = (on chiton) projection on tail valve (viii) demarking posterior from rest of valve. Varies in prominence and position.
myoglobin = red oxygen-binding protein in muscle tissue; often in buccal-mass muscles of gastropods. Similar to red haemoglobin in vertebrate blood, but green haemocyanin is usual oxygen-carrier in mollusc blood. See www.researchgate.net/publication/251227038_Radular_myoglo…
myostracum = microscopically thin discontinuous innermost layer of chiton valve.
nephridium (pl. nephridia) = cilia-lined excretory/osmoregulatory tubule (kidney).
nephridiopore = opening of nephridium for excretion. a.k.a. nephropore, or renal pore.
odontophore = firm, approximately ellipsoid, structure of cartilage supporting radula. Protruded like a tongue to operate radula. Often reddish from myoglobin, and medially grooved.
perinotum = dorsal surface of chiton’s girdle.
phototactic (adj.) = of species that moves towards light (positively phototactic) or away from it (negatively phototactic).
phototaxis = the positive (or negative) response of a freely moving organism towards (or away from) a light source.
plankton = animals and plants that drift in pelagic zone (main body of water). pleural area = triangular area on intermediate valve of chiton with its base along anterior edge of valve and its apex near the centre of the posterior edge. a.k.a. median triangle. Not visibly differentiated on A. fascicularis.
porcelaneous = resembling vitreous glazed ceramic material.
postmucronal = situated to posterior of mucro on tail valve.
properiostracum (on chitons) = proteinaceous material covering the shell. Different composition from periostracum of most other molluscs.
radula = ribbon of chitin bearing chitinous teeth that is extruded on a tongue-like odontophore of cartilage to rasp food. On chitons and limpets, teeth are usually impregnated with magnetite, a hard magnetic mineral of iron.
en.wikipedia.org/wiki/Radula
radular sac = tube, ending in a caecum, where radula is created and stored.
retrograde = (of locomotion waves on foot) waves travel from anterior to posterior.
sublittoral = below level of low water spring tide
tegmentum = outer shell-layer of chiton valves, usually porous and relatively soft. (Covered by properiostracum when live.)
tricuspid = having three points (cusps).
trochophore = spherical or pear-shaped larva that moves with aid of girdle of cilia. Stage preceding veliger, passed within gastropod egg in most spp. but free in plankton for limpets, Trochidae, Tricolia pullus and (with no veligers) chitons.
veliger = shelled larva of marine gastropod or bivalve mollusc which swims by beating cilia of a velum (bilobed flap). Stage may be passed in plankton or within liquid-filled egg-capsule.