Click image to enlarge with full caption. Main text below slider.
Crepidula fornicata (Linnaeus, 1758)
Meaning of scientific name: Crepidula = little slipper, fornicata = vaulted/arched.
Synonyms: Patella fornicata Linnaeus, 1758; Crepidula riisei Dunker, 1852; Crepidula virginica Conrad, 1871.
Current taxonomy: WoRMS https://www.marinespecies.org/aphia.php?p=taxdetails&id=138963
Vernacular: Slipper shell; Slipper limpet; Oyster-pest; Common Atlantic slippersnail; American slipper limpet; Devil’s toenails (English); Ewin mochyn; Ewin Mair; Brenigen wystrys (Welsh); Slippertje; Slipper; Muiltje; Gewoon muiltje (Dutch); Crépidule pontée; Crépidule; Berlingot de mer (French); Pantoffelschnecke (German); Tøffelsnegl (Danish); Ostronpest (Swedish).
Glossary below.
Shell Description
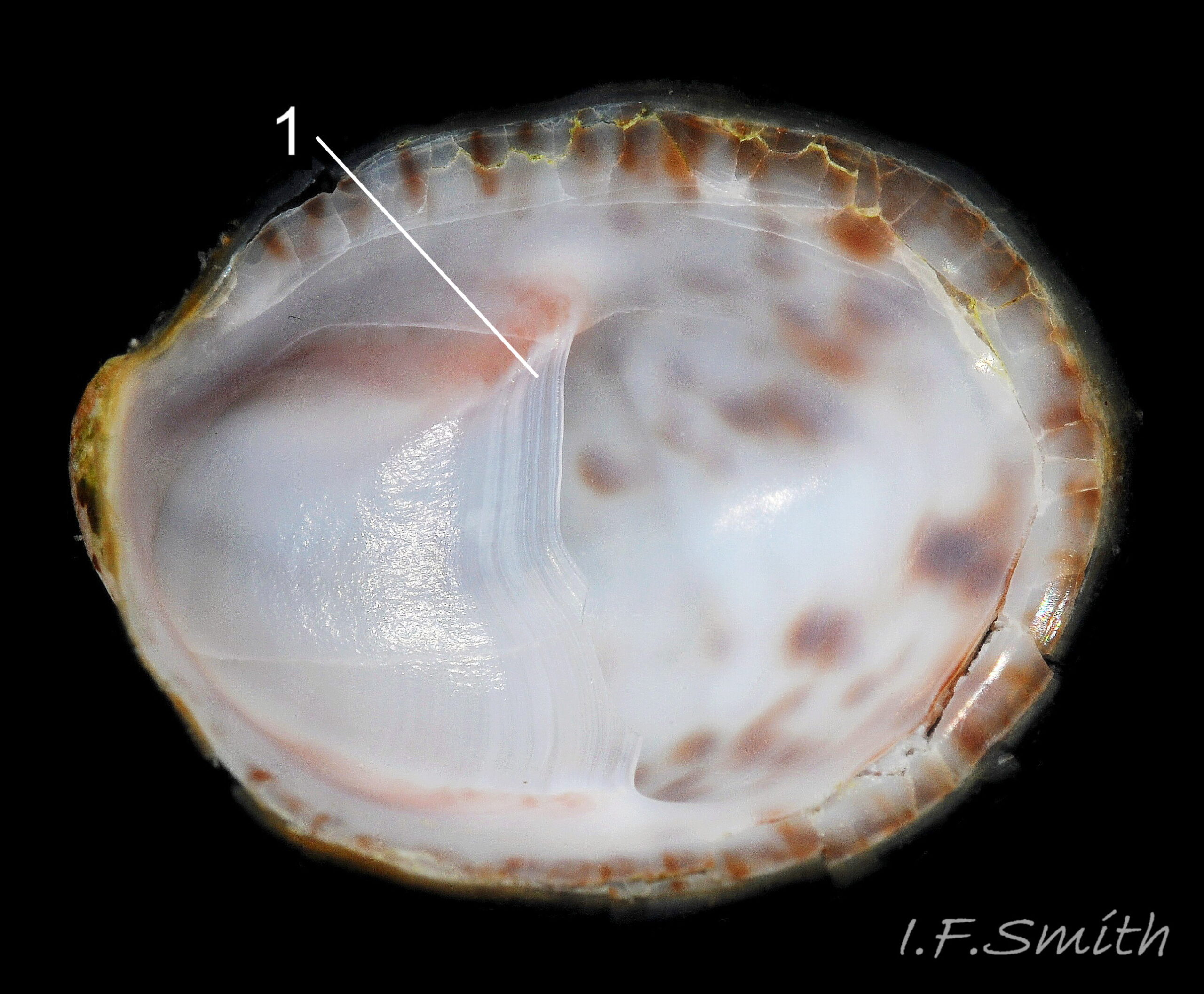
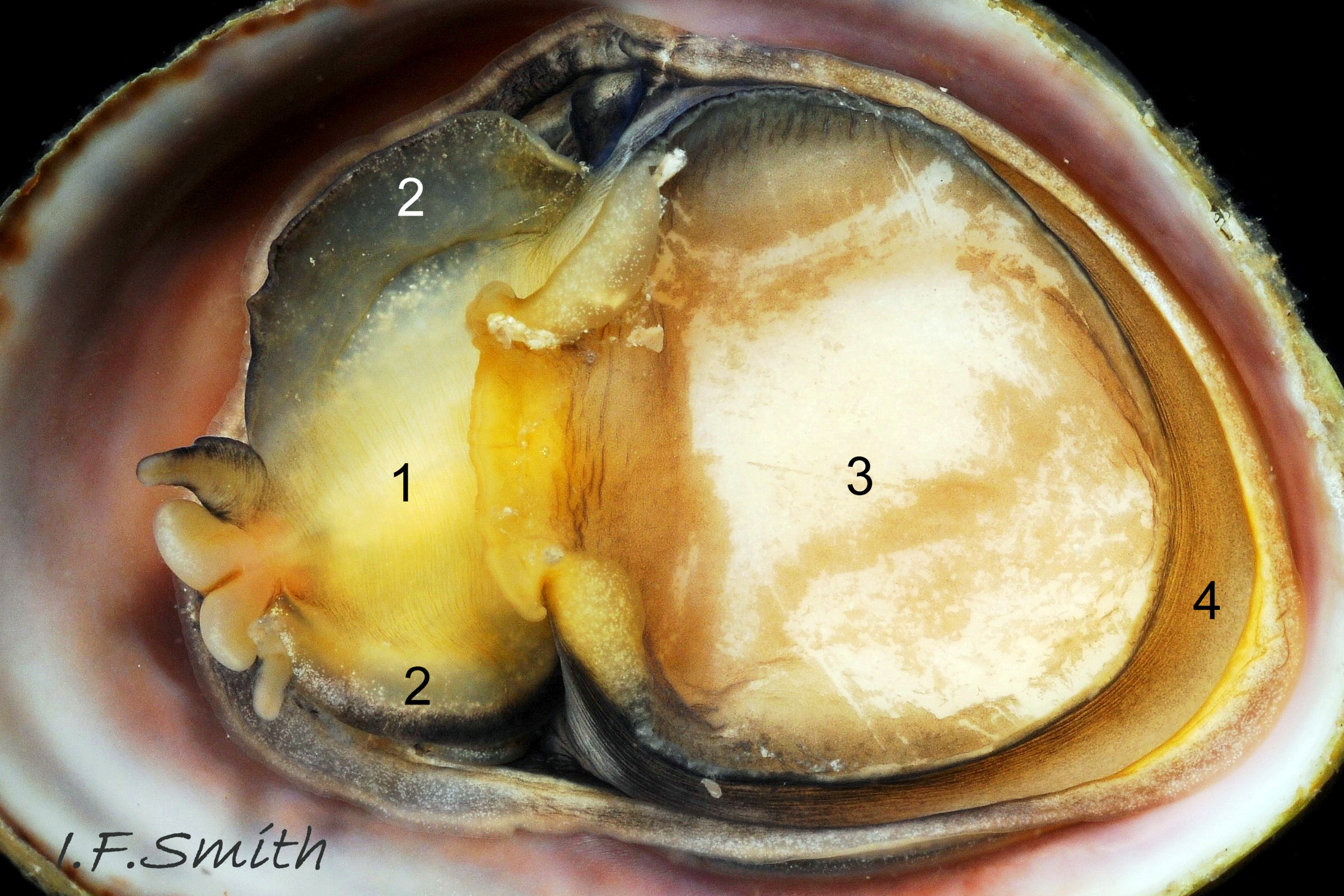
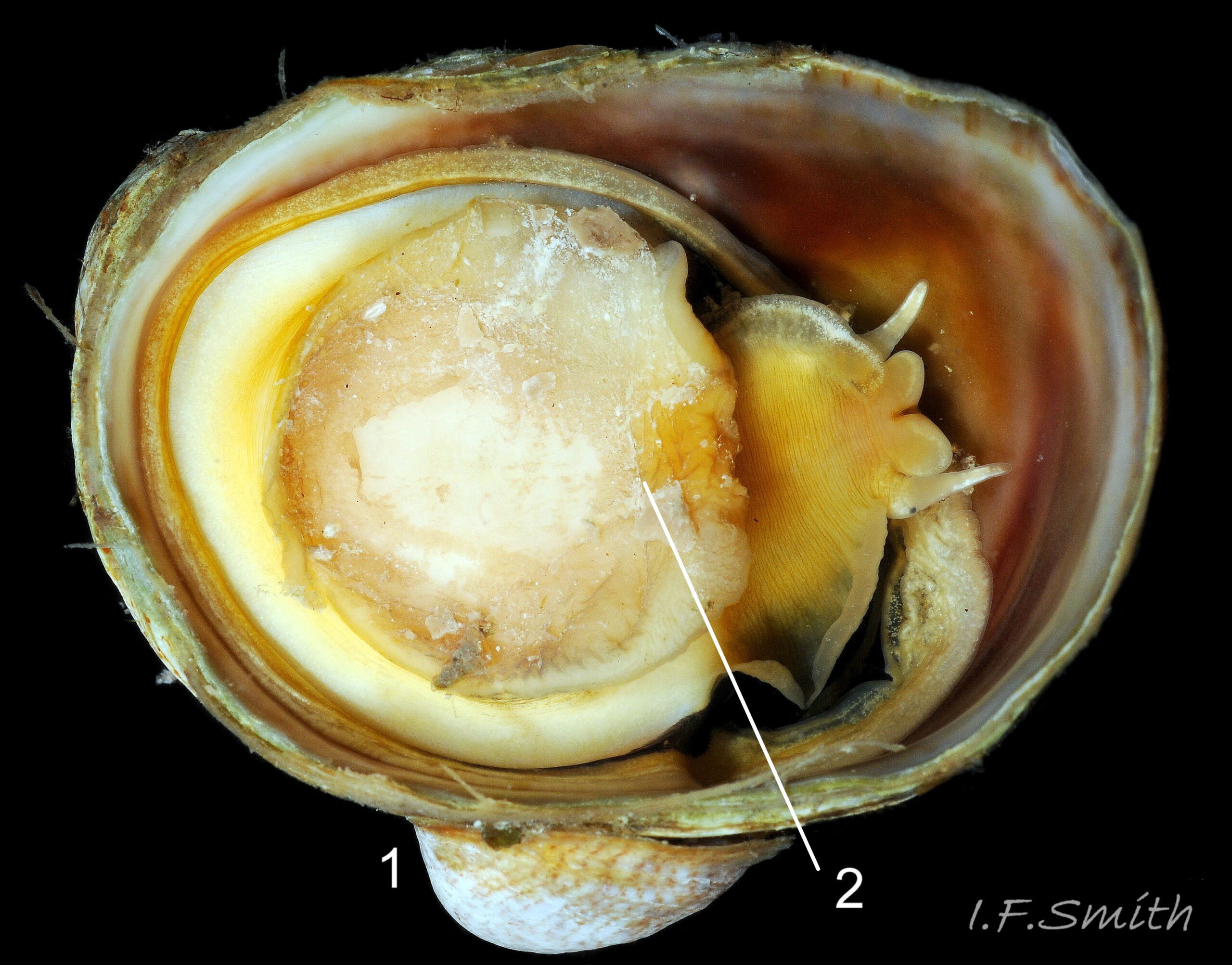
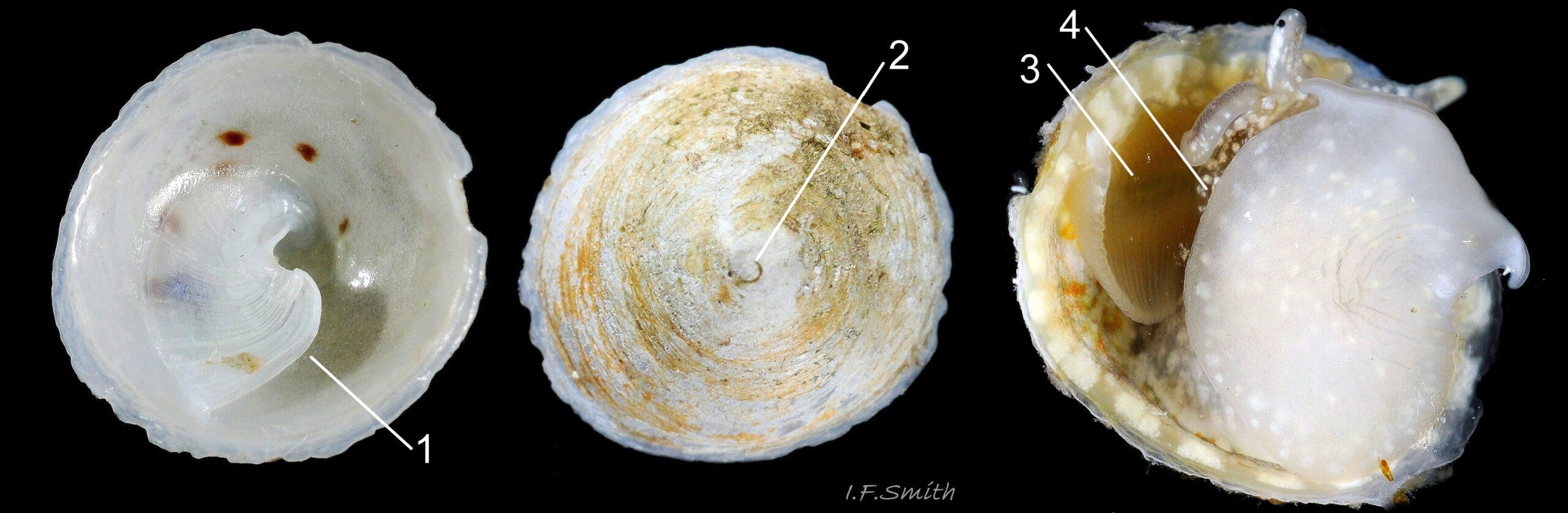
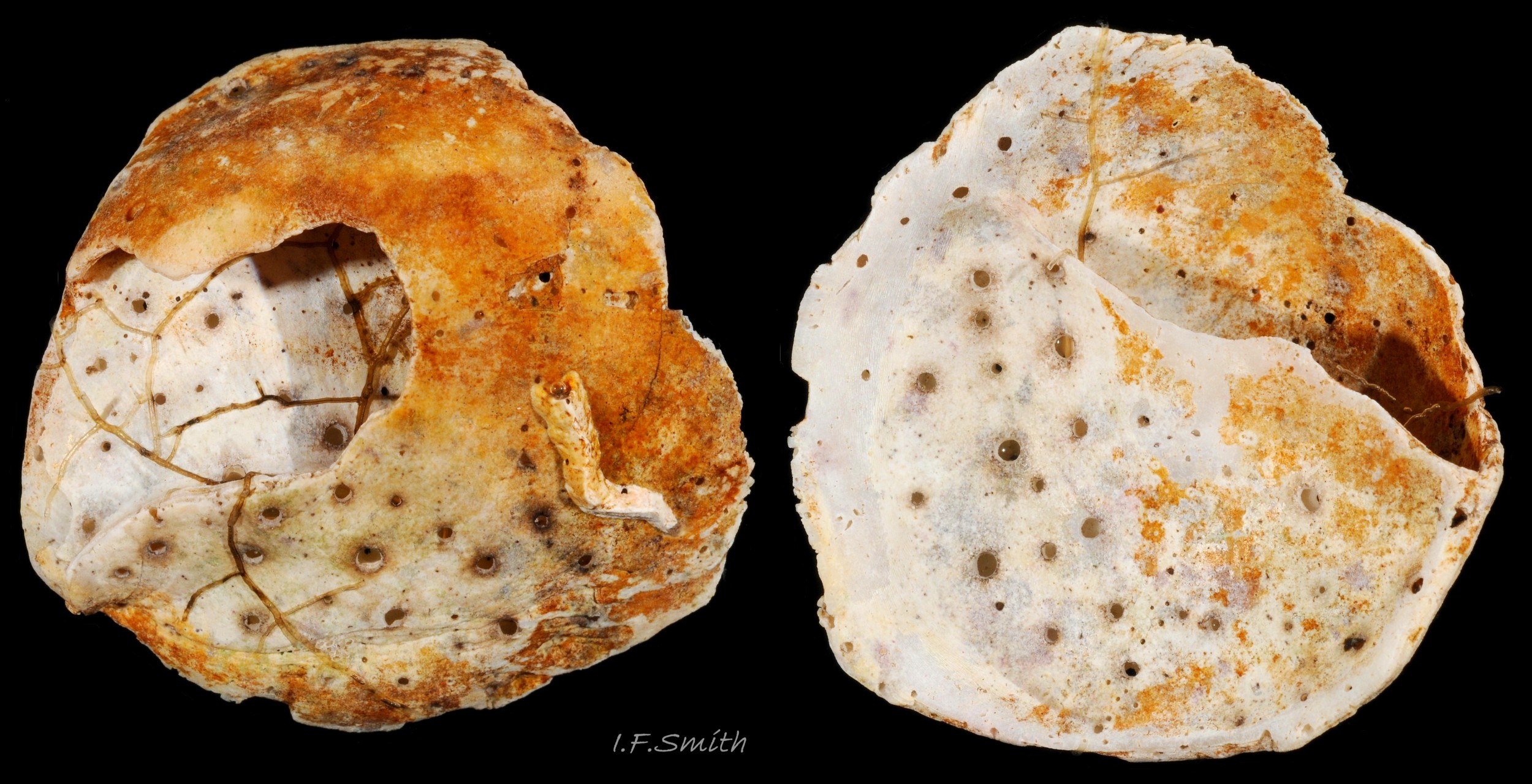
C. fornicata has a small depressed spire 01 Crepidula fornicata, a very large vaulted body whorl 02 Crepidula fornicata and a very large reniform aperture 03 Crepidula fornicata. The shell grows up to 50 mm long. It has no external sculpture apart from a few growth lines, and it is fairly glossy when young and unworn. The posterior half of the interior is partitioned by a large, transverse, shelf-like septum which nearly reaches the aperture lip 03 Crepidula fornicata. The shell is about 1.5 mm long when newly metamorphosed from the veliger larva. The septum is formed after metamorphosis of the veliger. At juvenile shell length 5 mm the septum is partly formed and tilted, and the shell interior is translucent, pearly white showing the brown marks of the exterior 04 Crepidula fornicata . When young, and usually male, the exterior is whitish or yellowish with thin wavy red-brown lines radiating from the spire, and there is often a white band on the periphery of the body whorl 01 Crepidula fornicata & 01.1 Crepidula fornicata . On older shells the exterior is often bleached and stained, but markings may be preserved in the shiny scar left when another adhering specimen is removed 05 Crepidula fornicata . The interior becomes tan or brown with age, apart from the septum which remains chalk-white with a brown edge where it meets the rest of the shell 03 Crepidula fornicata. The thin periostracum is usually fragmentary, yellow-brown and best seen where it extends beyond the rim of the aperture when young 01 Crepidula fornicata; it is darker when older 05 Crepidula fornicata . The larval operculum does not persist on adults.
Body Description
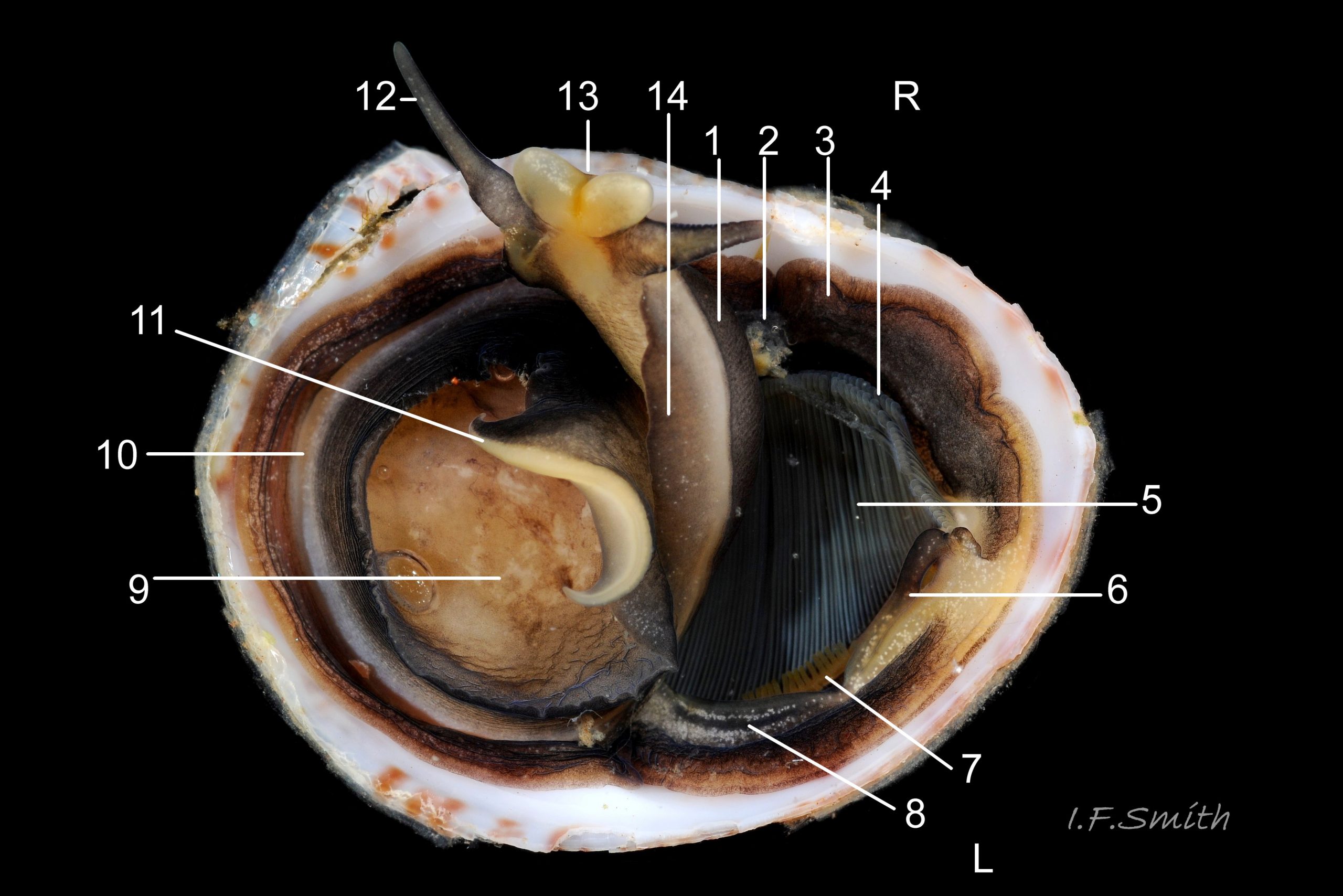
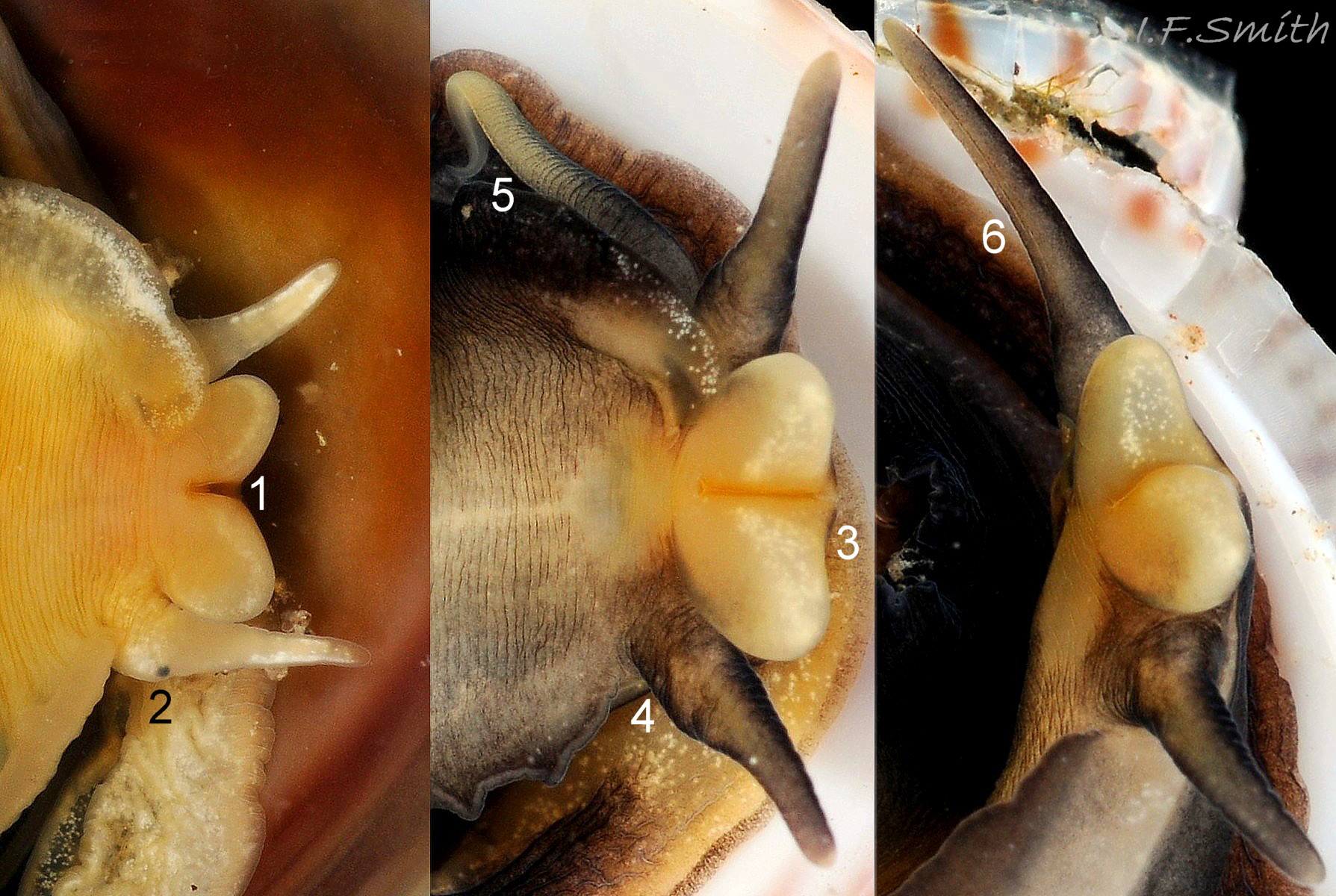
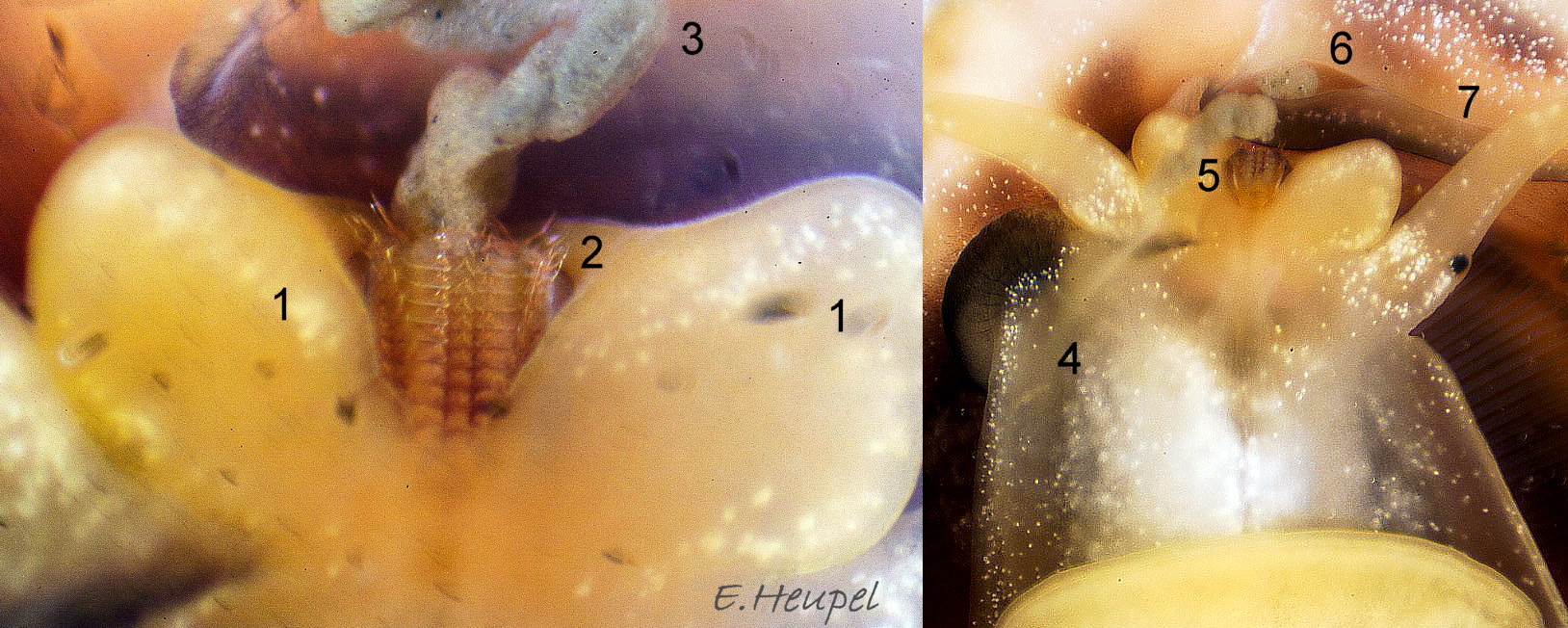
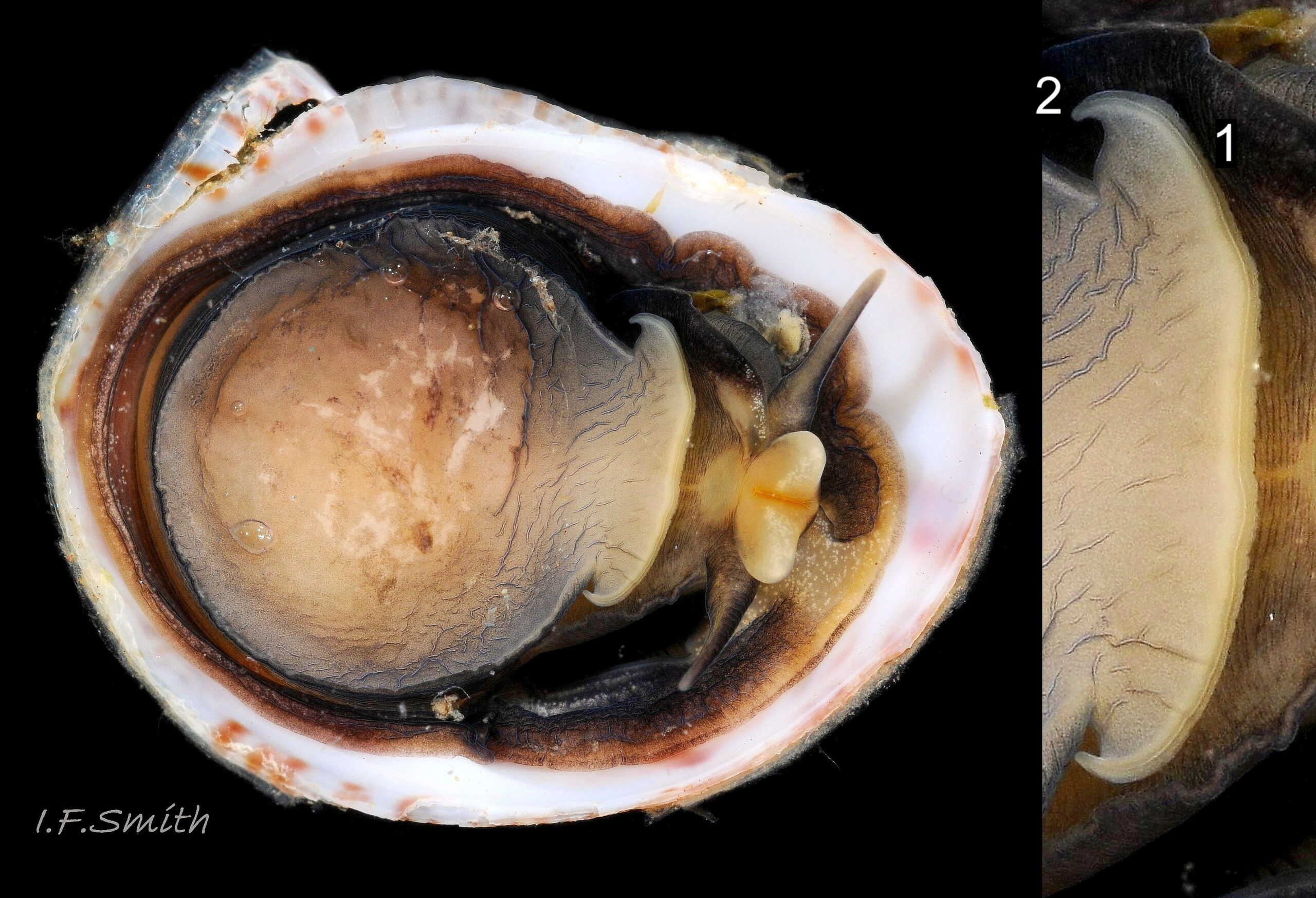
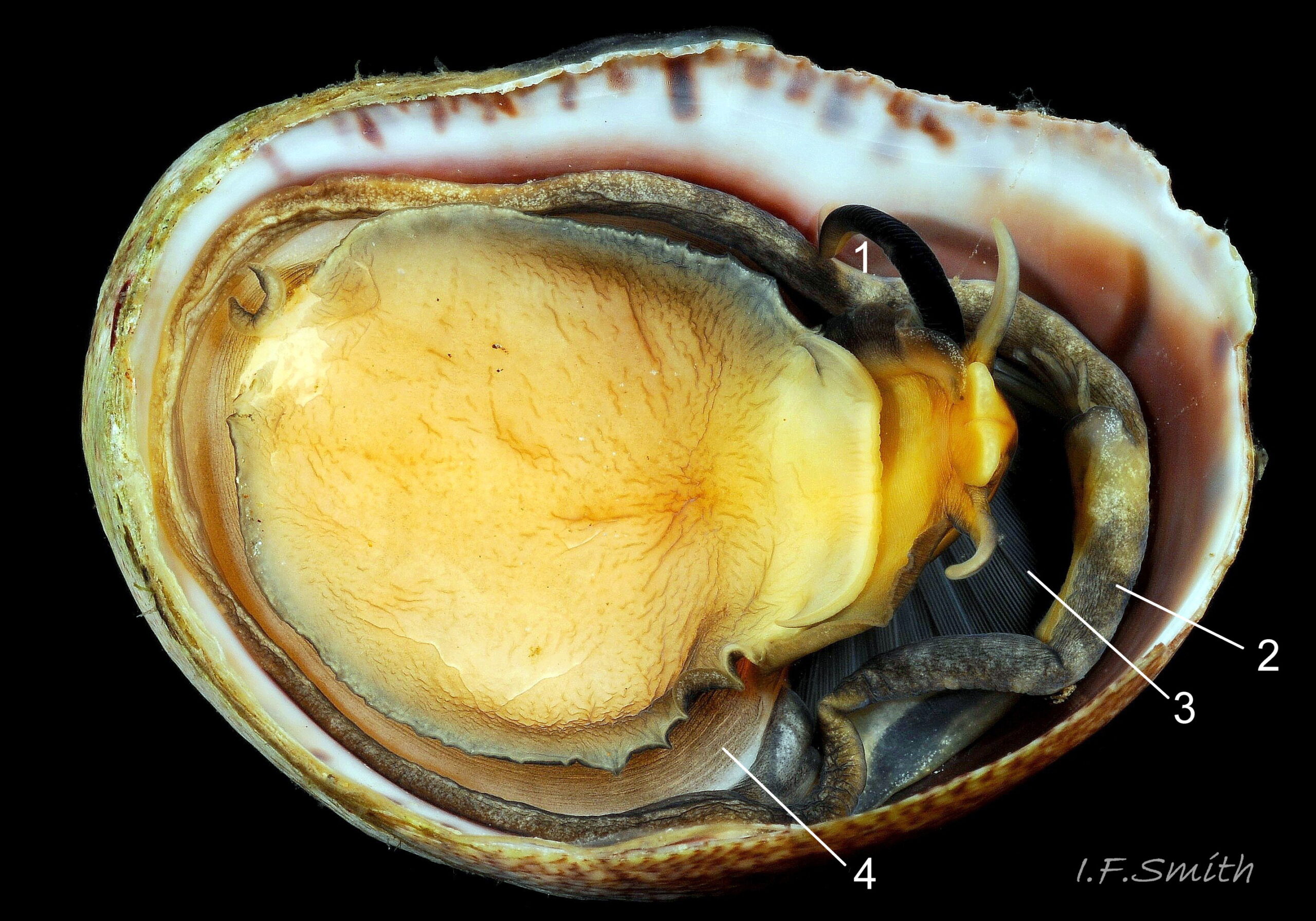
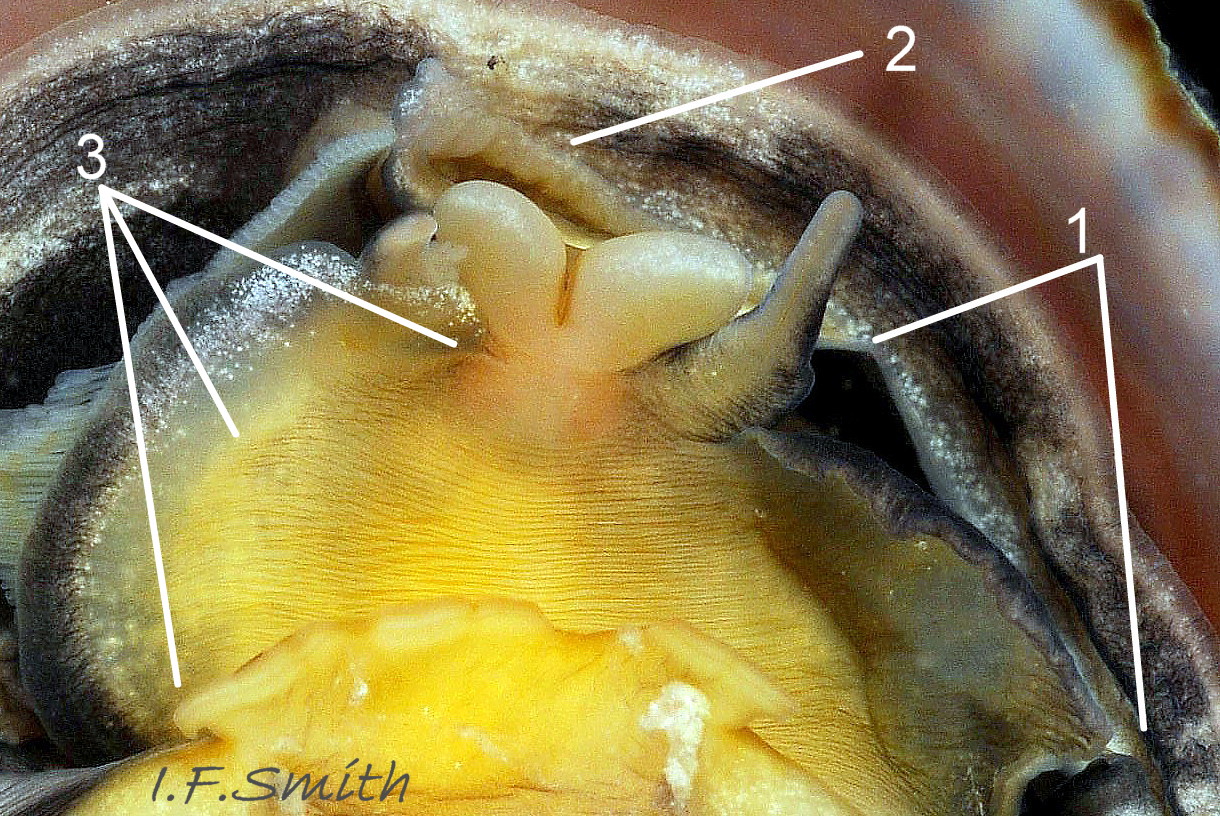
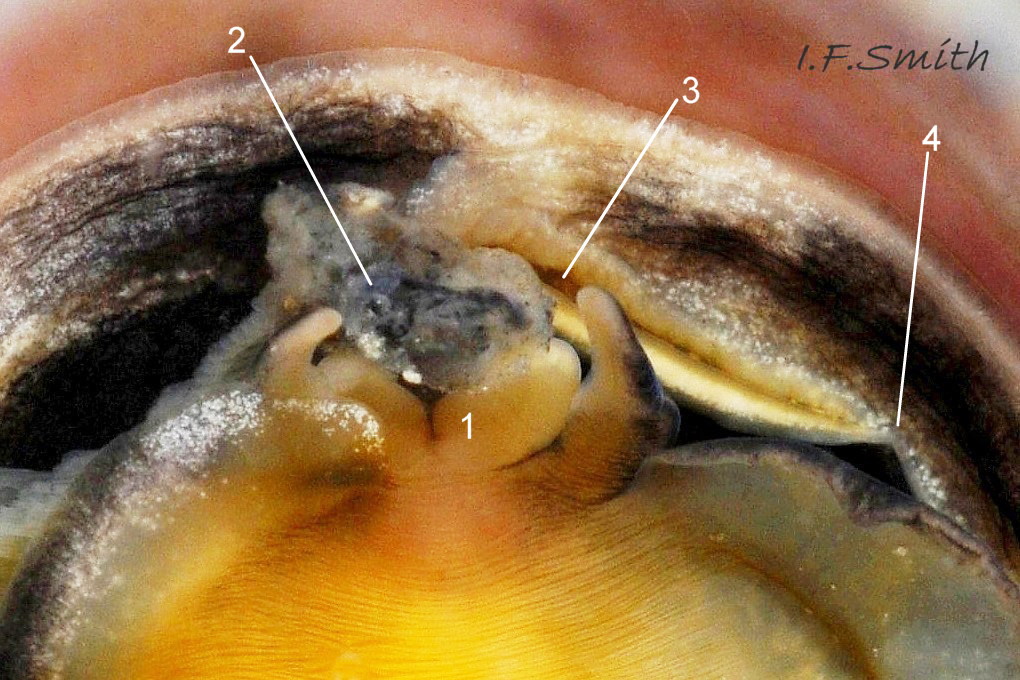
The flesh is yellowish with variable, often extensive, amounts of blackish pigment 06 Crepidula fornicata. The great majority of larger adults are dark; pale ones are usually small. The snout usually lacks blackish pigment ventrally. It consists of two distinct lobes separated by a groove 07 Crepidula fornicata which opens when the radula protrudes 09 Crepidula fornicata. The teeth on the radula are needle-like, suited to manipulating soft mucus-food rolls rather than rasping or sweeping food. The cephalic tentacles have a thickened base with a distinct eye spot when the tentacle is pale, but it is indistinct when the tentacle is dark 07 Crepidula fornicata. Dorsally the tentacles are transversely wrinkled when contracted, and often darker than the ventral face which has a longitudinal medial crease. Behind the tentacles, the head is elongated and expanded. When extended, the area of the cephalic mass, including the substantial neck-lobes, a.k.a. cephalic wings, is c. 50% that of the foot 08 Crepidula fornicata. The anterior of the body is often dark dorsally 06 Crepidula fornicata, but paler ventrally 08 Crepidula fornicata. The horseshoe shaped pedal retractor muscle 06 Crepidula fornicata is attached to the septum of the shell 03 Crepidula fornicata .
The foot is almost circular with a distinct, flexible anterior extension (propodium) 10 Crepidula fornicata . The translucent edge beyond the pedal retractor muscle is often darkened by the dark dorsal surface. The propodium has a bilaminate anterior edge with small angled lateral extensions. The mantle colour varies from yellowish-white 11 Crepidula fornicata to dark chestnut 06 Crepidula fornicata with little or much black and grey. A pallial filter consisting of a porous sheet of mucus stretches from the mantle skirt to the left, inhalant, side of the body. The filter is usually lost when C. fornicata is dislodged. A pallial food groove 06 Crepidula fornicata and food pocket 09 Crepidula fornicata & 13 Crepidula fornicata are set into the anterior-left of the mantle skirt.
A large, deep mantle cavity occupies the anterior half of the shell 12 Crepidula fornicata. A monopectinate ctenidium, many times larger than usual on gastropods 06 Crepidula fornicata, has greatly elongated filaments hanging from the roof of the mantle cavity with their tips resting on a food groove on the floor of the cavity. The male has a long, transversely wrinkled penis arising from the base of the right cephalic tentacle and tapering to a thin tip. The wrinkles are smoothed when it is extended to reach a female lower in the stack of individuals. It can be all black 12 Crepidula fornicata or a combination of yellowish and grey 07 Crepidula fornicata. The female has a pallial oviduct formed from the vas deferens of the preceding male phase; some females have a vestigial penis.
Key identification features
Crepidula fornicata
1) Reniform vaulted shell with small depressed spire at one end 01 Crepidula fornicata.
2) Reniform aperture with internal septum 03 Crepidula fornicata.
3) Parts of body black 06 Crepidula fornicata.
4) Very large blackish ctenidium 06 Crepidula fornicata.
Similar species
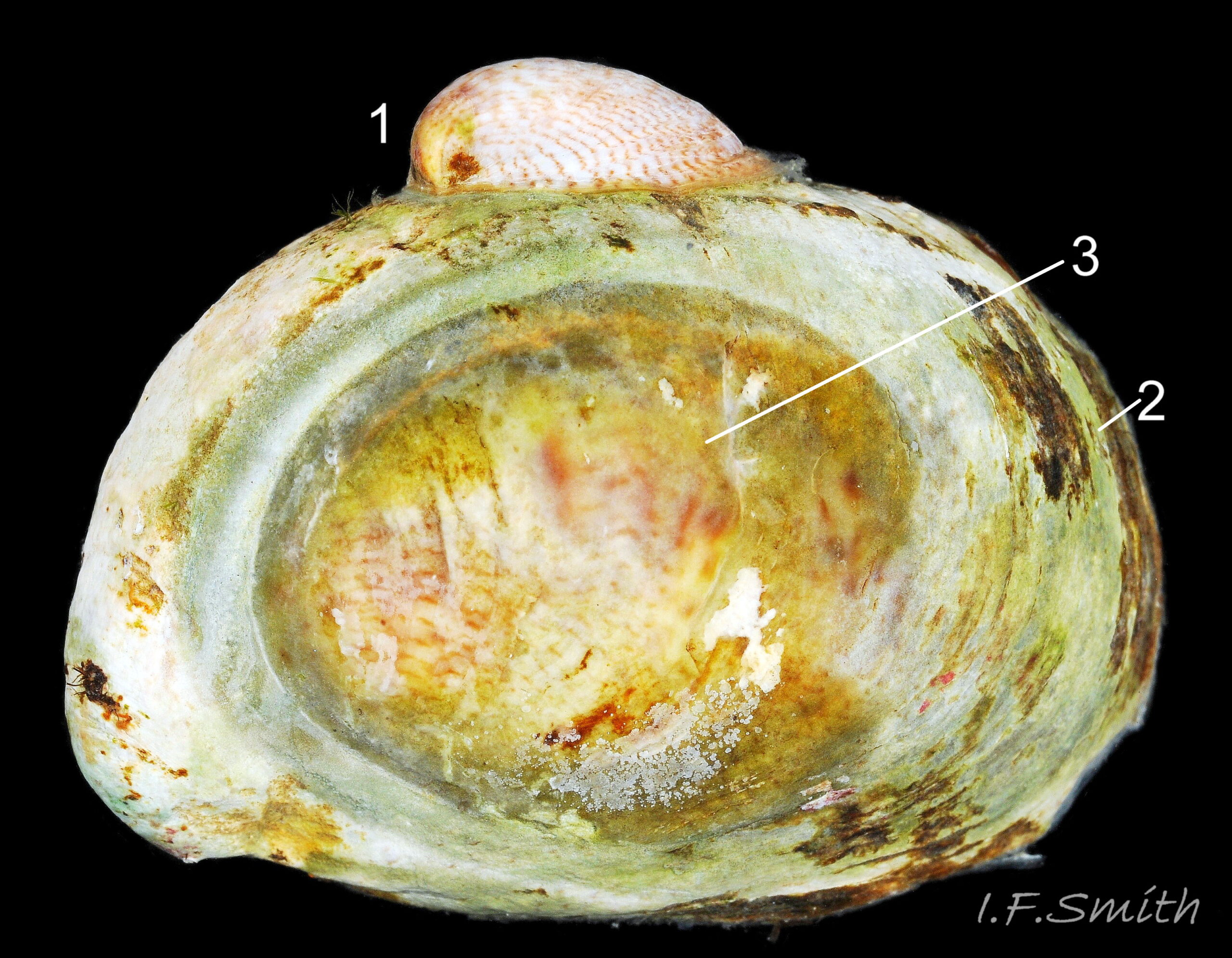
Calyptraea chinensis (Linnaeus, 1758) 13.1 Crepidula fornicata
1) Patelliform cone with nearly central apex.
2) Nearly circular aperture with internal septum.
3) Body translucent white, fawn-white or grey-white with flakes of opaque white.
4) Very large whitish or yellow-white ctenidium.
Patella spp. and other limpets.
2) Conical shell with no internal septum (shelf).
4) No ctenidium (Patella spp.) or small ctenidium (some other limpets).
Habits and ecology
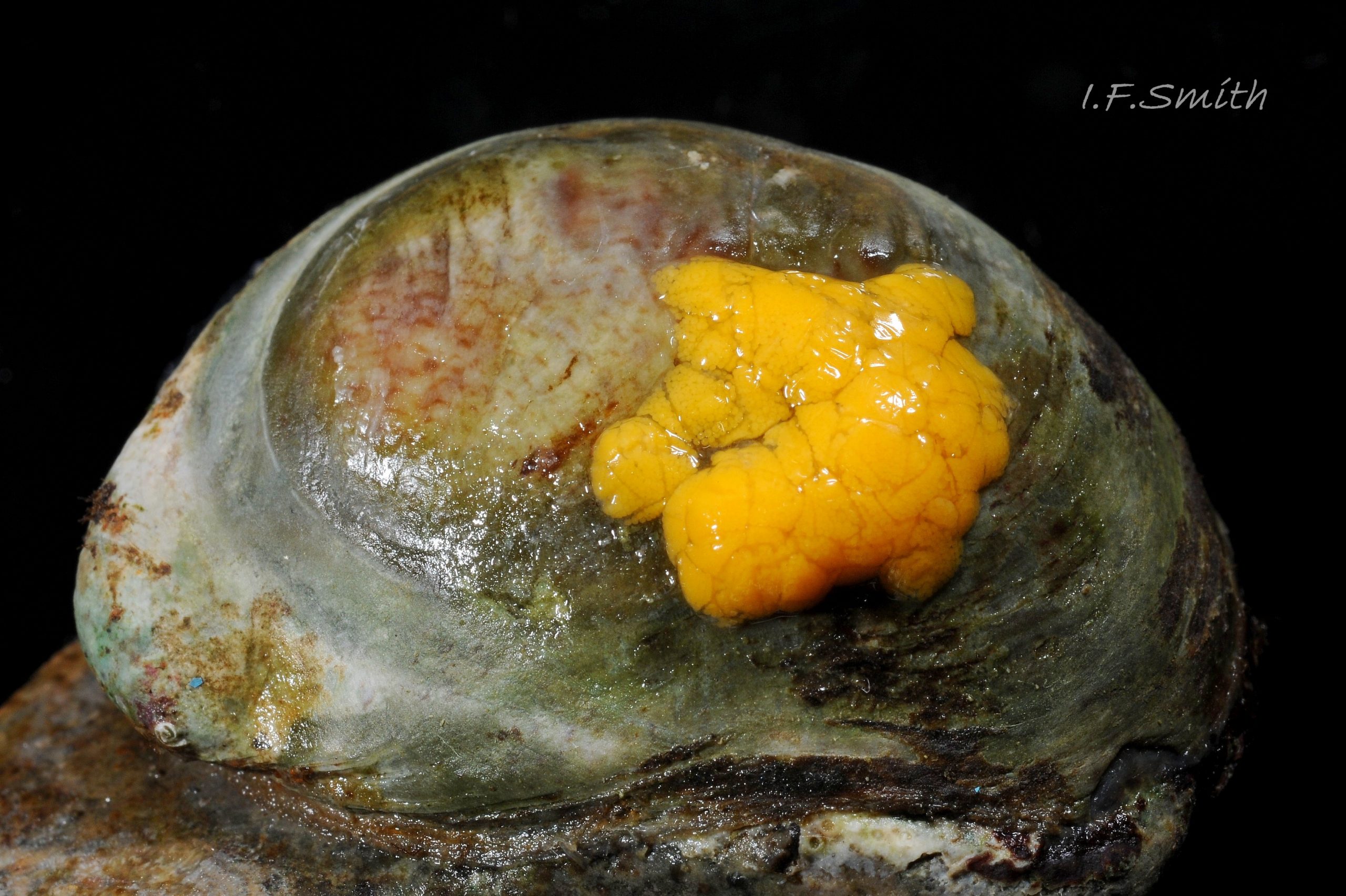
C. fornicata lives mainly sublittorally on hard and soft substrates to 10 m, but sometimes on stony beaches at LWS, and often stacks of specimens are thrown onto the shore by waves. It tolerates salinity down to 25‰. It can live singly or in stacks of up to 15 individuals on top of each other, on shells and stones 14 Crepidula fornicata. Live oysters are especially favoured as initial attachment sites as the environmental conditions ideal for oyster growth seem to be the optimum for C. fornicata growth and reproduction. It also settles on live Pecten maximus, Mytilus edulis and Buccinum undatum 22 Crepidula fornicata. It may benefit from the feeding current of underlying bivalves. When the substrate is soft sediment, C. fornicata selects a position raised clear of it to avoid clogging of the ctenidium. It grips the substrate, other mollusc or another C. fornicata with its sucker-like foot, perhaps assisted by adhesive secretions as the force needed to dislodge them sometimes results in damage to the foot 11 Crepidula fornicata. Except for the early male stage, C. fornicata is immobile and unable to reattach to the substrate if dislodged. In such cases, the basal animal dies, but others in the stack above live on using the basal shell as substrate. Dislodged individuals leave a shiny, but not excavated, adhesion mark 05 Crepidula fornicata .
C. fornicata is one of only a handful of British gastropods which feed exclusively like bivalves by filtering phytoplankton and suspended organic particles from the water; it is one of the most perfectly adapted to this mode of life (Fretter & Graham 1962). Others are Calyptraea chinensis, Turritella communis, Capulus ungaricus, Viviparus contectus and Bithynia tentaculata. A strong transverse, left to right, respiratory current is created by cilia covering the very large ctenidium 06 Crepidula fornicata . The current carries in large amounts of food material.
A web of mucous threads forms a pallial filter across the entrance to the mantle cavity to the left of the head. Large food particles are caught by it from the inhalant current and moved by cilia along a deep pallial groove, which protects the food from contamination by faeces, to a food pocket in the mantle where it is stored 15 Crepidula fornicata. When required, the radula licks food into the mouth 09 Crepidula fornicata. There is minimal wear on the needle-like teeth of the radula as the diet is entirely soft. Smaller food particles pass through the pallial filter to the ctenidium where they are caught by the branchial filter consisting of porous sheets of mucus coating both sides of the ctenidium. The sheets of mucus are constantly growing and being driven with attached food down the ctenidium to the ciliated food track on the floor of the mantle cavity where they are shaped into rolls. Cilia carry the rolls along the track across the base of the right neck-lobe to the mouth 09 Crepidula fornicata. Unlike the pallial groove, the food track is exposedto occasional faecal contamination.
Defence against attack is provided by the shell and by repugnatorial glands embedded in the mantle edge, which give off repellent protein in persistent threads when violently stimulated (Fretter & Graham, 1962). The tiny, pyramidellid gastropod Boonea seminuda is a parasite of Crepidula fornicata (Fretter & Graham, 1962) in America https://www.gbif.org/species/2298496 .
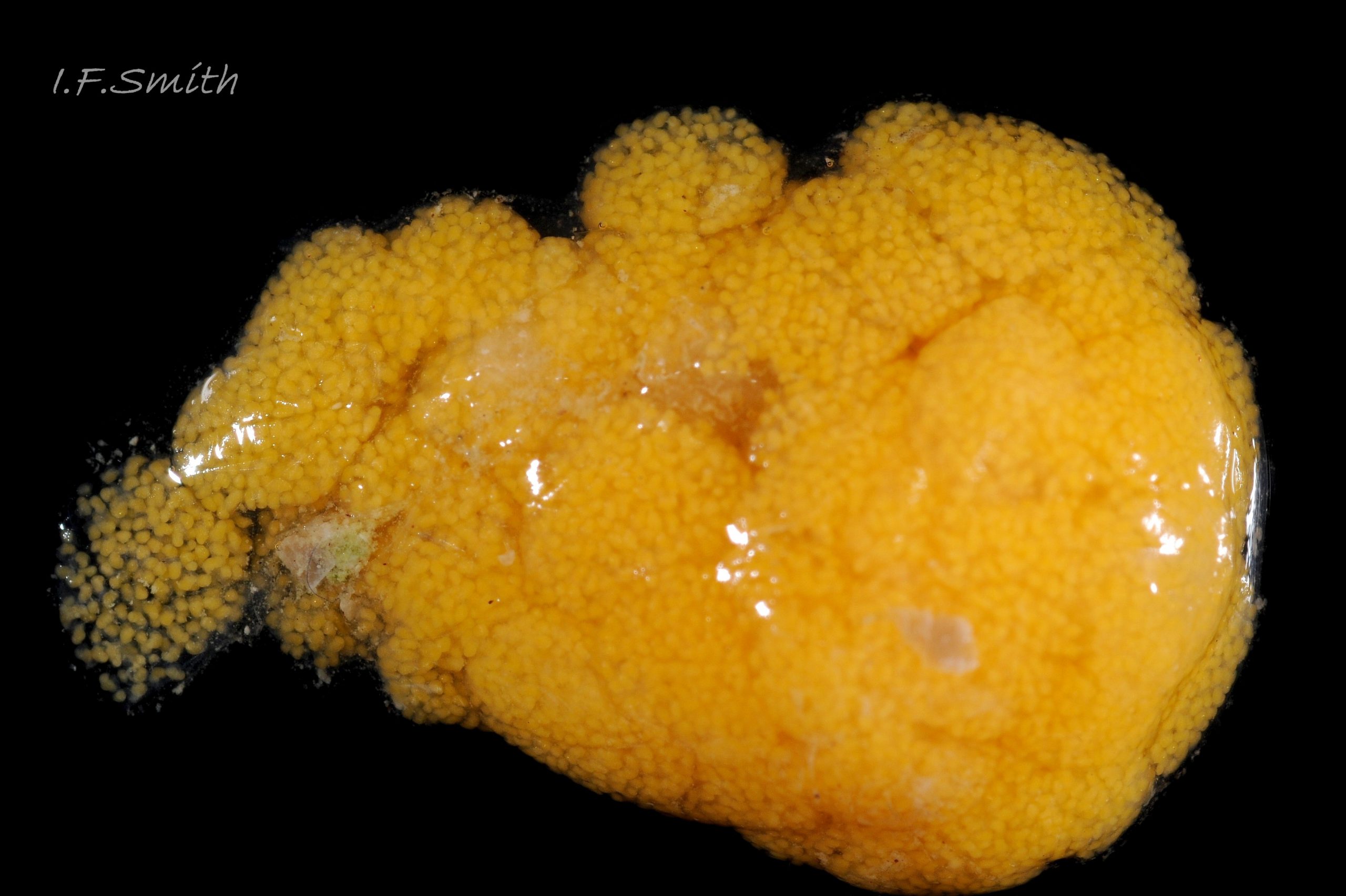
Breeding: the small male uses a long extendible penis 12 Crepidula fornicata to reach the pallial oviduct of a female lower in the stack of individuals 14 Crepidula fornicata. Females spawn twice a year from April to October in Britain. Very soft, thin-walled, stalked, 2-4 mm at widest point, capsules contain 250-300 orange ova each in a common mass of albumen 16 Crepidula fornicata. The female holds each capsule for a time before attaching up to 70 by stalks to the substrate under the anterior part of her shell in the course of the strong inhalant current 17 Crepidula fornicata & 18 Crepidula fornicata. The female incubates them until free swimming veliger larvae escape into the plankton. Eventual settlement and metamorphosis to adult form is stimulated by proximity of other C. fornicata. All young are born as males, as C. fornicata is a protandrous sequential hermaphrodite. Those settling in isolation soon change to female, but those on older females initially function as males, usually changing sex in their third summer. Then remaining sperm is absorbed, the penis reduced and gradually lost, and the open-groove vas deferens closes to form a pallial oviduct. Often the animals in the middle of a stack are undergoing sex change and may show both male and female features in the same individual such as a reduced penis and spawn at the same time. Sex change is not always at the same development stage and is affected by environmental factors, such as starvation or proximity of other individuals.
C. fornicata is a serious impediment to oyster cultivation in Europe. Overwhelming numbers, 1750/m² recorded in France, filter out suspended food before oysters can access it, cause habitat deterioration with their faeces and prevent oyster spat from settling with their strong feeding currents. To recover former oyster beds from blankets several centimetres deep of C. fornicata in some creeks in southern England, 50 tonnes/hectare of C. fornicata were removed, but the beds were just as badly overwhelmed again within ten years.
Distribution and status
C. fornicata is a native of east coast North America, currently living from Nova Scotia to Mexico. It has spread with commercial movement of oyster stock to other parts of the world such as the Pacific coast of North America and Japan, and especially Europe where it is well established, sometimes abundantly, from Southern Britain and Kattegat (not inner Baltic) to the Mediterranean; GBIF map https://www.gbif.org/species/5192789 .
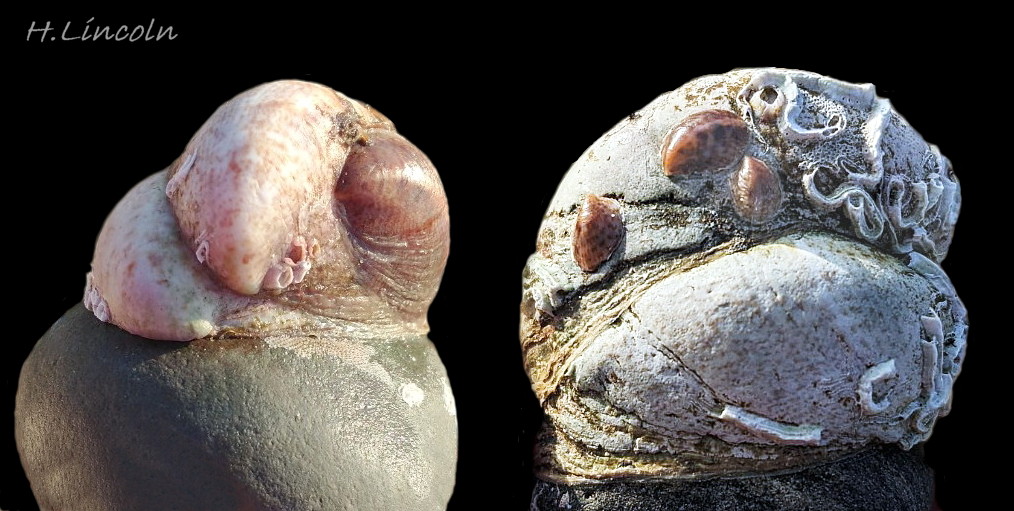
Graham (1988) gave the British distribution as south coast England, originating from introduction with imported oysters in 1887-1890 to Essex. Scattered live and dead records elsewhere such as Bristol Channel, Northumberland, Northern Ireland and Kerry were attributed to probable importation with oysters, and Graham expressed doubt about their ability to establish permanent breeding populations as the earliest introduction at Liverpool, 1872, and several others around Britain, had failed. But in the years since 1988 it has established breeding populations on the east coast up to the Humber and on the west coast to Pembrokeshire and probably beyond. In North Wales, there have been occasional records, often of long dead shells 19 Crepidula fornicata, which may be failed introductions. Following finds of a live stack of three on a Buccinum undatum in February 2020 near oyster cultivation in the Menai Strait and another find in November 2020 in SW Anglesey near the mouth of the Menai, Natural Resources Wales found it frequently in 2021 (P. Brazier, pers. comm., November 28, 2023) and records submitted to iRecord in 2023 with substantiating photographs of live breeding stacks from Llŷn, Arfon, west Anglesey and the Menai Strait show that it is now well established in North Wales 20 Crepidula fornicata & 21 Crepidula fornicata. UK map verified by Conchological Society of GB & Ireland: https://records.nbnatlas.org/occurrences/search?q=lsid:NBNSYS0000174750&fq=data_provider_uid:%22dp117%22&nbn_loading=true#tab_mapView
Links and references
Fretter, V. and Graham, A. 1962. British prosobranch molluscs. London, Ray Society.
Graham, A. 1988. Prosobranch and pyramidellid gastropods. London.
iRecord https://irecord.org.uk/help
Werner, B. 1955 Uber die Anatomie die Entwicklung und Biologie des veligers und der Veliconcha von Crepidula fornicata. Helgoländer wissenschaftliche Meeresuntersuchungen 5: 169–217. https://hmr.biomedcentral.com/articles/10.1007/BF01610508#citeas
Yonge, C.M. and Thompson, T.E. 1976. Living marine molluscs. London.
Acknowledgements
For use of images and information I gratefully thank Paul Brazier (Natural Resources Wales), Dave Hernon, Eric Heupel (University of Connecticut, U.S.A), Harriet M. Lincoln (Natural Resources Wales) and Adam Parker.
Glossary
a.k.a. = ‘also known as’
aperture = mouth of gastropod shell; outlet for head and foot.
branchial = of or relating to branchiae (gills/ctenidium).
cephalic = (adj.) of or on the head.
cilia = (pl.) vibrating linear extensions of membrane used in feeding or locomotion. (“cilium” singular).
ciliated = (adj.) coated with cilia.
ctenidium = comb-like molluscan gill; usually an axis with a row of filaments either side.
ELWS = extreme low water spring tide (usually near March and September equinoxes).
left = (of animals) ‘left’ & ‘right’ refer to sides of animal when viewed dorsally with head at top of frame; most live images of C. fornicata are ventral views.
mantle = (a.k.a. pallium) sheet of tissue which secretes the shell and forms a cavity for the ctenidium.
monopectinate = (of ctenidium) filaments only on one side of axis/rachis.
odontophore = cartilaginous “tongue” which supports and extends/retracts the radula.
operculum = plate of horny conchiolin, rarely calcareous, used to close shell aperture.
pallial = (adj.) of, relating to, or produced by the mantle (pallium).
pallium = (a.k.a. mantle) sheet of tissue which secretes the shell, covers the viscera and forms a cavity in gastropods.
patelliform = shaped like the cone of limpets in genus Patella.
pedal retractor muscle = large muscle which connects the foot to the septum of the shell.
periostracum = thin horny layer of chitinous material often coating shells.
plankton = animals and plants that drift in pelagic zone (main body of water).
propodial = (adj.) at the front of the foot.
propodium = anterior part of foot.
protandrous = (of sequential hermaphrodites) born as male, changes later to female.
protogynous = (of sequential hermaphrodites) born as female, changes later to male.
radula = ribbon of chitinous teeth extruded on a tongue-like structure (odontophore) to rasp food.
reniform = kidney-shape outline.
right = (of animals) ‘left’ & ‘right’ refer to sides of animal when viewed dorsally with head at top of frame; soft-part images of C. fornicata are ventral views.
septum = partitioning wall.
setose = bearing many setae.
seta = stiff hair or bristle. (pl. setae)
vas deferens = duct which carries sperm to penis.
veliger = shelled larva of marine gastropod or bivalve mollusc which swims by beating cilia of a velum (bilobed flap).
r – shelled larva of marine gastropod or bivalve mollusc which swims by beating cilia of a velum (bilobed flap).