Click image to enlarge with full caption. Main text below slider.
Patella ulyssiponensis Gmelin, 1791.
PDF available at www.researchgate.net/profile/Ian_Smith19/research
Current taxonomy: World Register of Marine Species (WoRMS)
www.marinespecies.org/aphia.php?p=taxdetails&id=140684
Synonyms: Patella aspera Lamarck,1819 (widely used by many authors until 1970s but now considered by WoRMS to be separate Macaronesian species P. aspera Röding, 1798); Patella_athletica Bean, 1844; P. depressa auct.
Jeffreys (1865) mistakenly took the rudimentary description of P. depressa by Pennant (1777) to be what is currently (2015) called P. ulyssiponensis. Until 1923, most authors followed Jeffreys in applying the name P. depressa Pennant to the wrong species, and in using the name P. intermedia Jeffreys for what is now recognised as P. depressa Pennant. Examination of Pennant’s type specimen by Tomlin (1923) exposed the error and authors started to use the name P. athletica Bean, 1844, for what is now called P.ulyssiponensis but, probably to avoid confusion, many retained use of P. intermedia Jeffreys for the true P. depressa Pennant, despite Pennant’s priority, until the 1970s (e.g.Yonge & Thompson, 1976).
Vernacular names: China limpet (English); Brenigen dorfelen (Welsh); Ruwe schaalhoren (Dutch);
Meaning of name: Patella (Latin) = little pan, ulyssiponensis (Latin) = from Lisbon.
Terms in text used with restricted or specialised meaning are marked with hashtag#; refer to
GLOSSARY below.
Shell Description
Patellid limpets have great geographical variation within and between species; this account refers to British specimens.
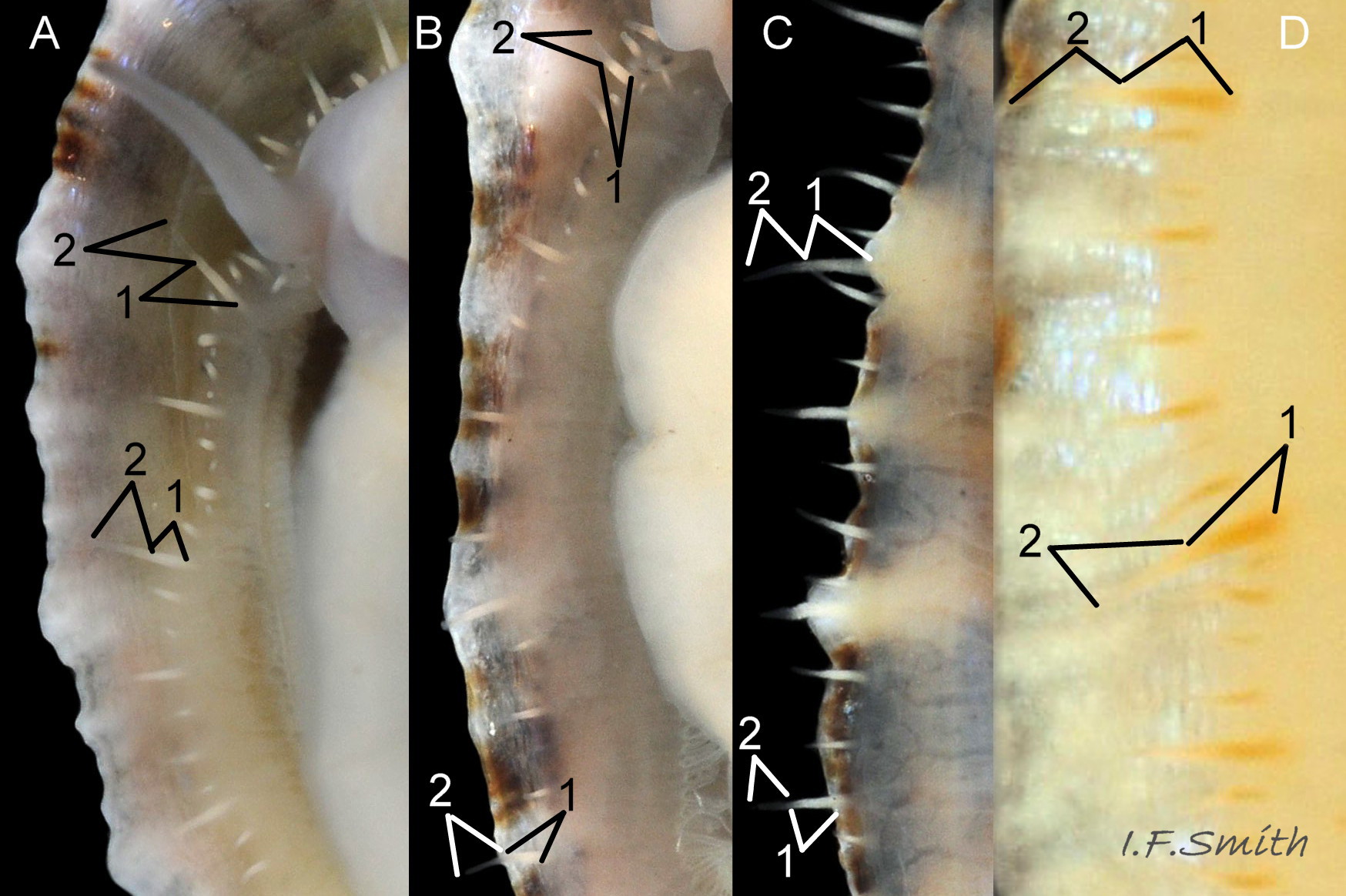
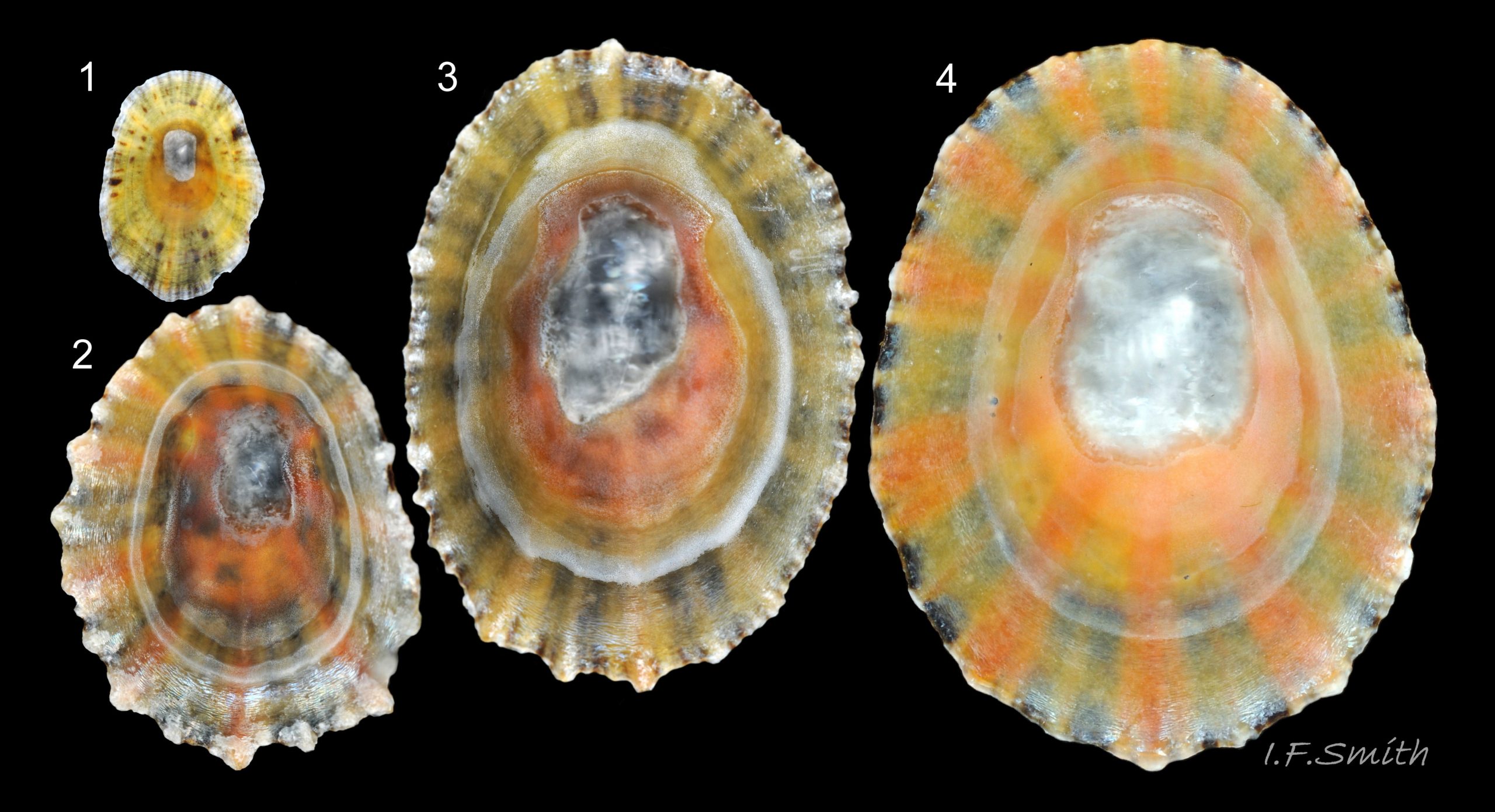
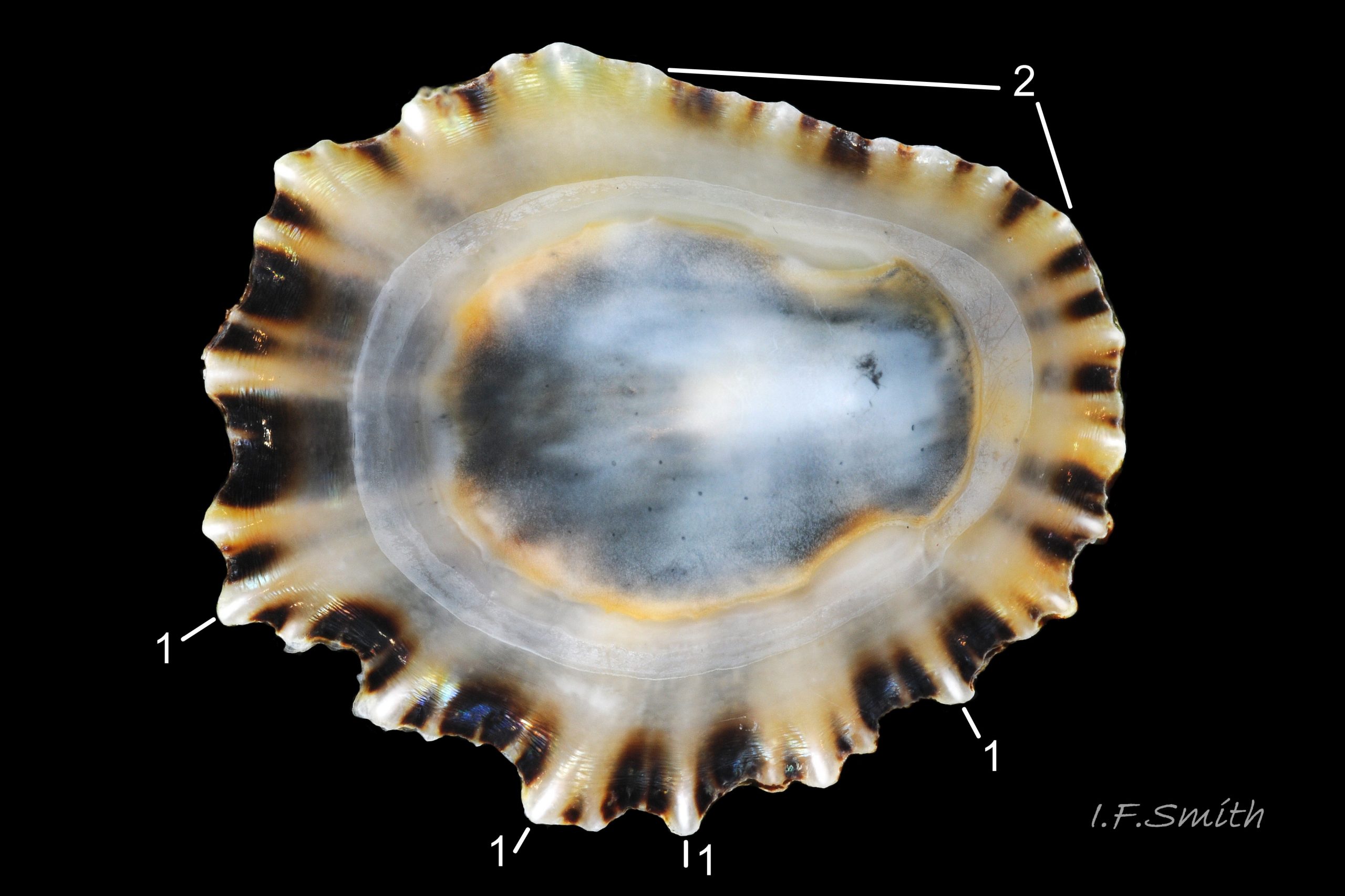
Up to 50mm long and 20mm high 01 Patella ulyssiponensis. Strong. Conoid; apex to anterior of centre, base ovoid, widest and sometimes angulated at posterior. Profile usually low (H/L 18-34%, sample of 18) 02 Patella ulyssiponensis & 03 Patella ulyssiponensis. In profile, anterior and posterior straight 04 Patella ulyssiponensis, varying to slightly convex 03 Patella ulyssiponensis or slightly concave 05 Patella ulyssiponensis . When not eroded, often sculpture of single, narrow, pale radiating ribs with varying numbers of intervening weaker ribs 06 Patella ulyssiponensis but often obscured by algal growths 07 Patella ulyssiponensis & 08 Patella ulyssiponensis. Sometimes ribs project from aperture-rim as points 09 Patella ulyssiponensis & 10 Patella ulyssiponensis, but frequently eroded down 11 Patella ulyssiponensis. Dark radiating rays between strong ribs often coalesce into bands 11 Patella ulyssiponensis.
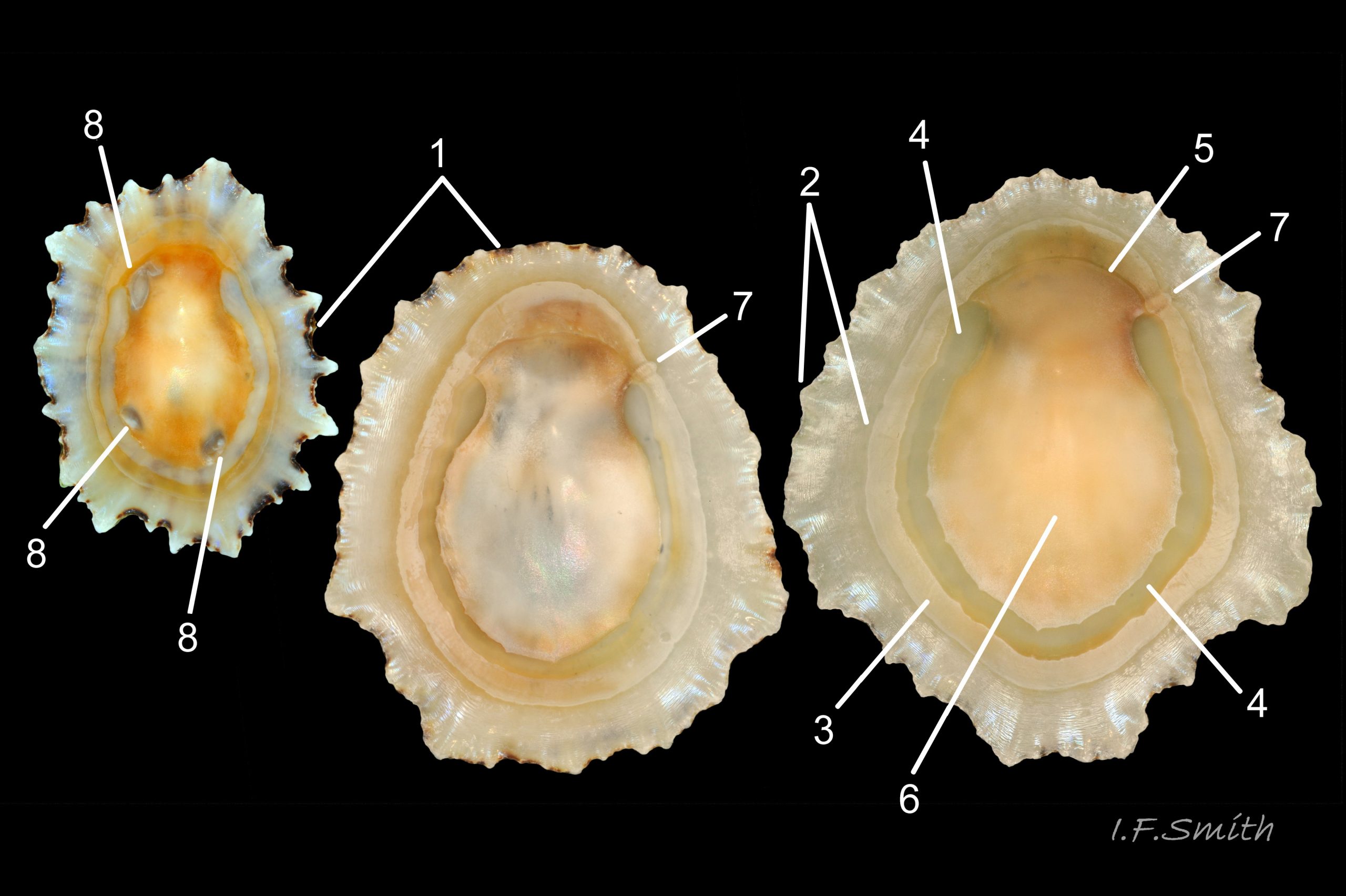
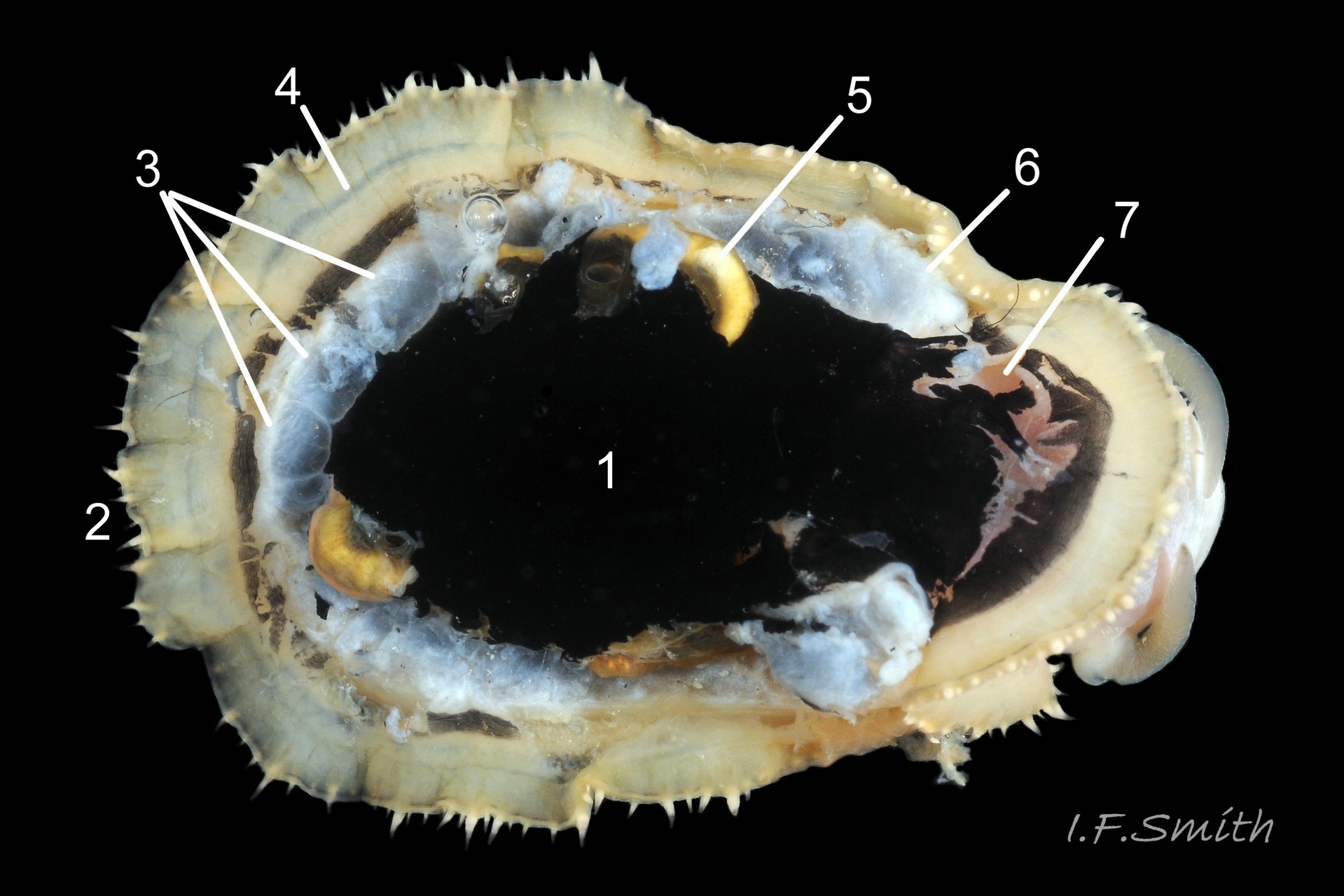
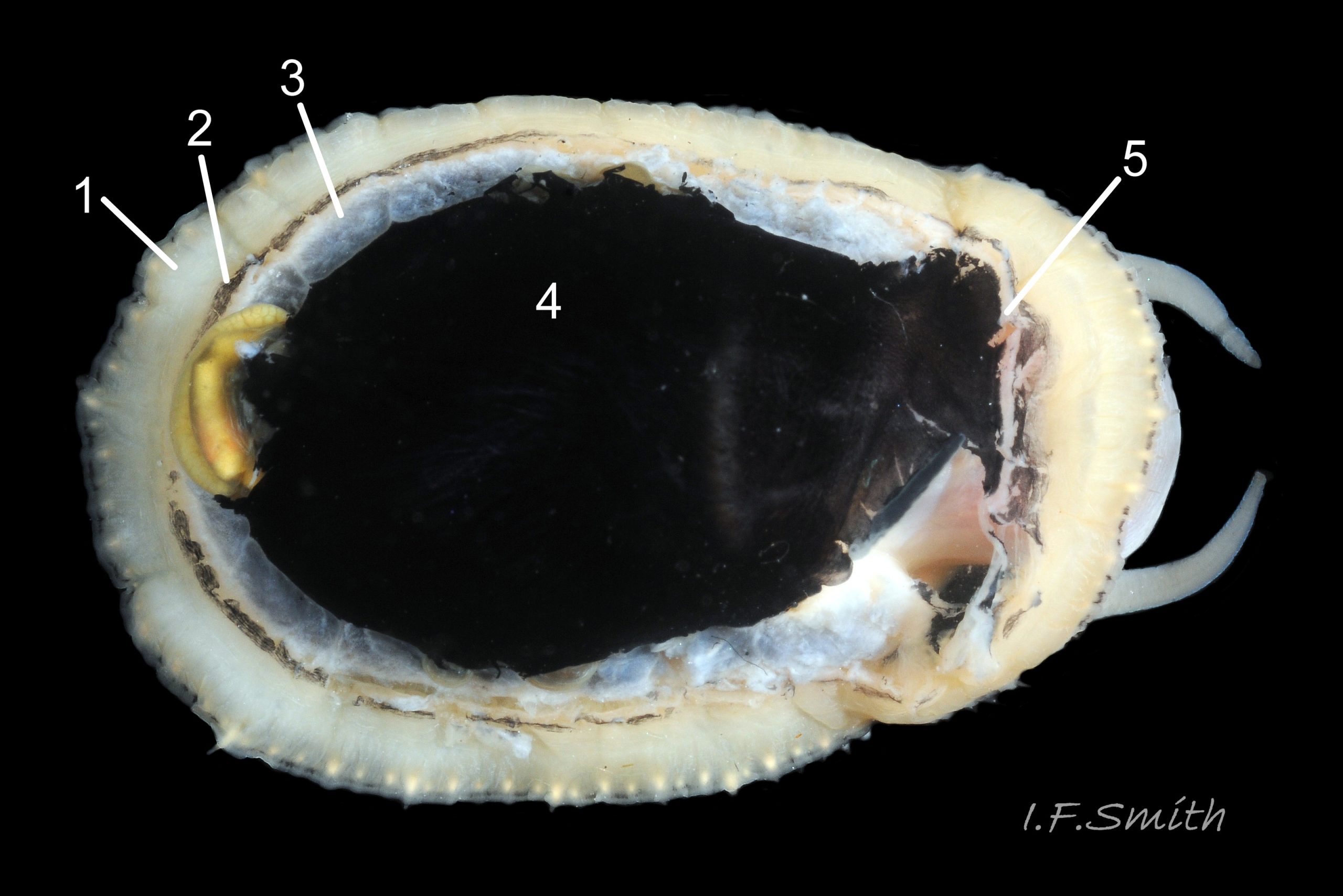
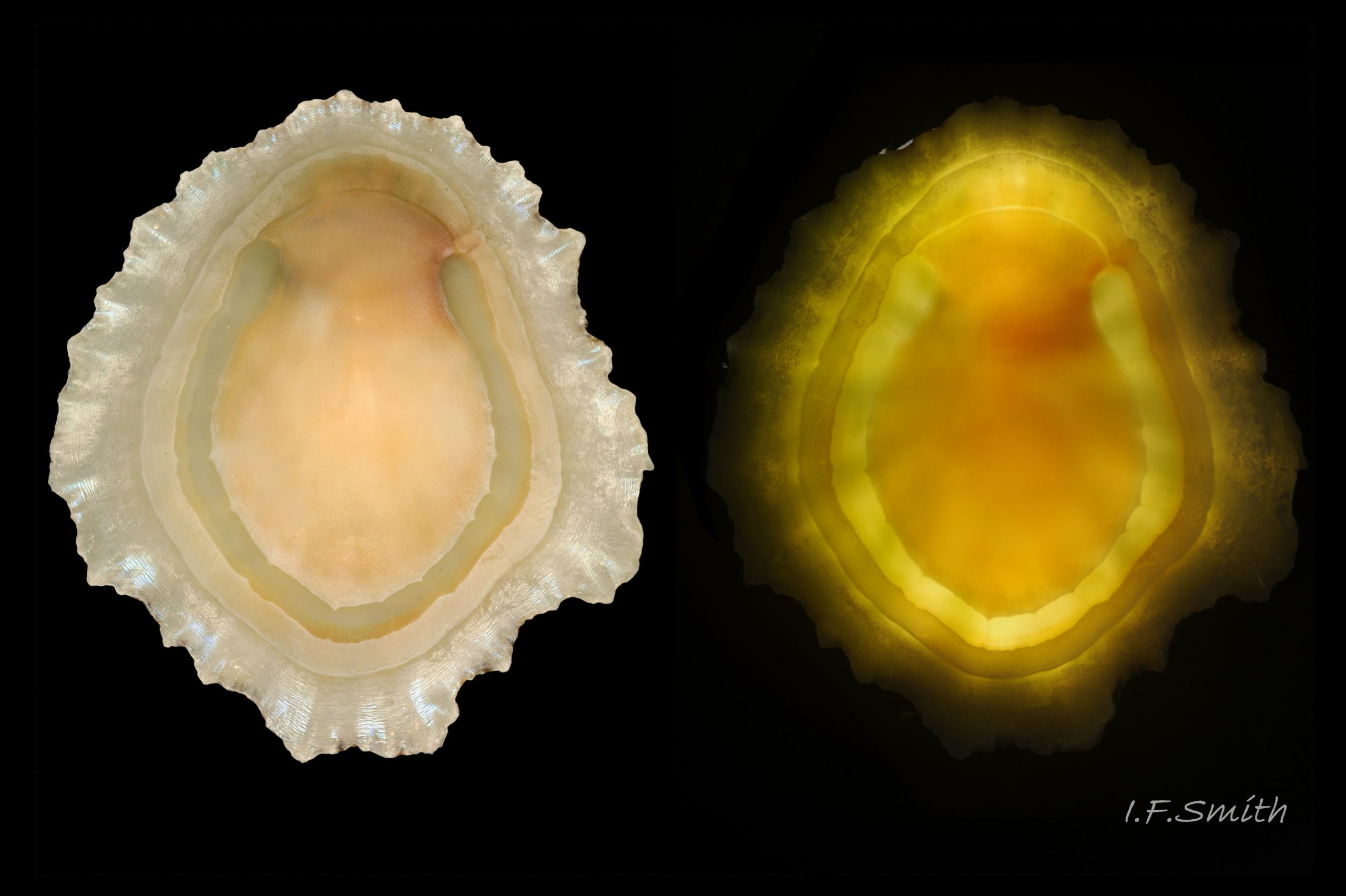
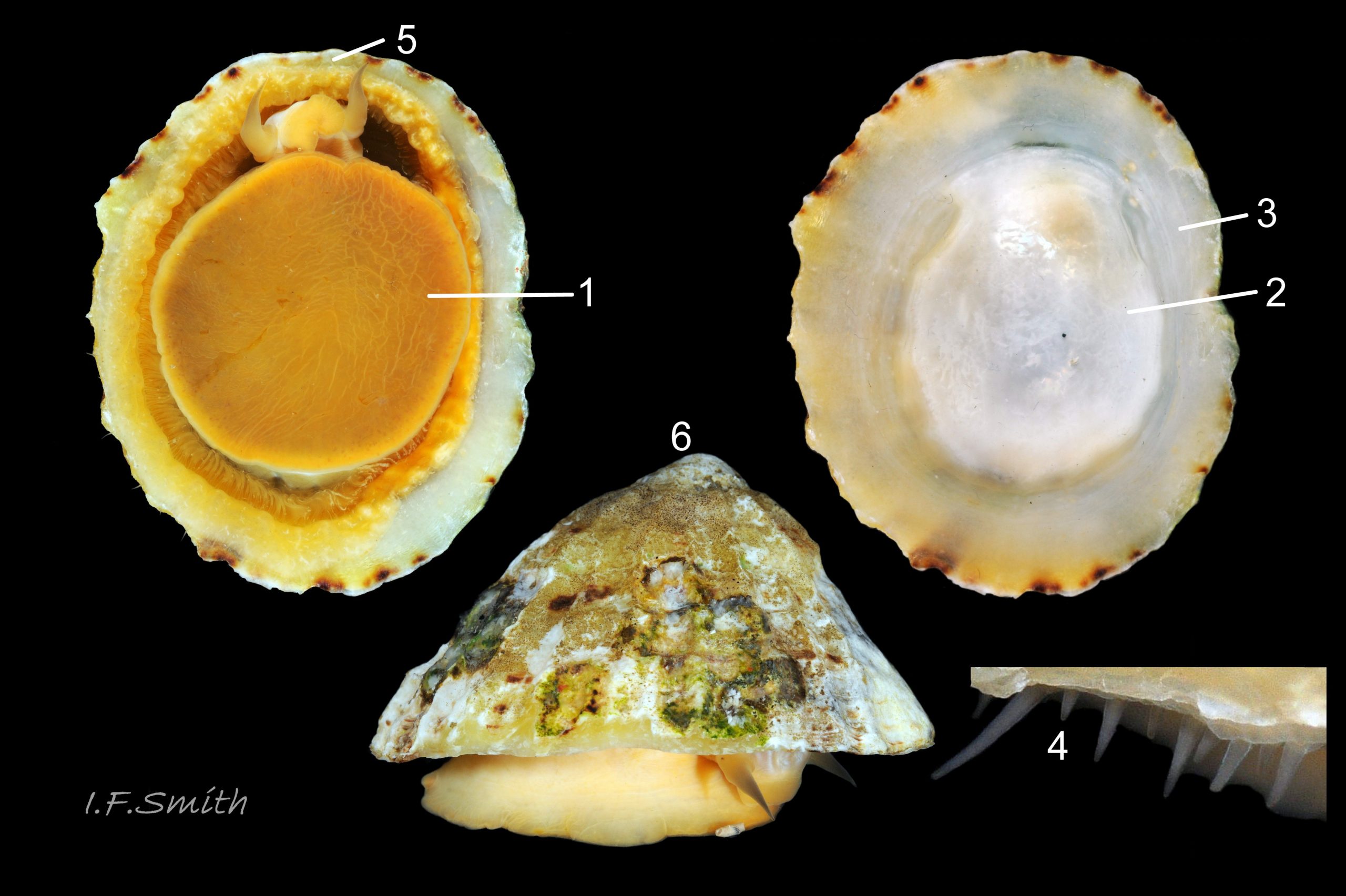
Most published descriptions and illustrations are confined to specimens with well-developed, thick, porcellaneous layers on the shell-interior e.g. www.conchsoc.net/spAccount/patella-ulyssiponensis . Some of this form show features defined by differences of orange staining 14 Patella ulyssiponensis and/or reflectivity 01 Patella ulyssiponensis resulting from differences in crystal-form of shell-material made by different parts of mantle. Following regions may then be recognised: 1) aperture rim, minute part (or none) of pigmented exterior shell-layer; secreted by mantle-edge, 2) wide peripheral “skirt layer” that reflects light, often iridescing blue, from many short crystalline lines parallel to rim; secreted by mantle-skirt, 3) narrower, matt, opaque, “pallial groove-band”; secreted by mantle roofing groove that contains gills, 4) translucent, horseshoe-shape “pedal-retractor muscle scar”; mark left by muscle attachment, 5) very thin “anterior mantle-attachment scar” connecting ends of pedal-retractor scar; mark left by mantle attachment, 6) central “amphora area” enclosed by scars 4 & 5; secreted by mantle over visceral hump, 7) short mark across pallial groove-band where efferent pallial vessel enters nuchal cavity through gap in pallial gills. On this form, interior layers conceal outer shell so, internally, “marginal rays are never conspicuous” (Fretter and Graham, 1994).
However, on some shores , e.g. in north Yorkshire, majority have thin inner layers revealing exterior colour rays 02 Patella ulyssiponensis & 11 Patella ulyssiponensis) . On these, the structural features 1-7 described above are usually indiscernible or ill-defined, apart from frequently orange-stained amphora area 13 Patella ulyssiponensis and iridescent lines in skirt-layer 15 Patella ulyssiponensis.
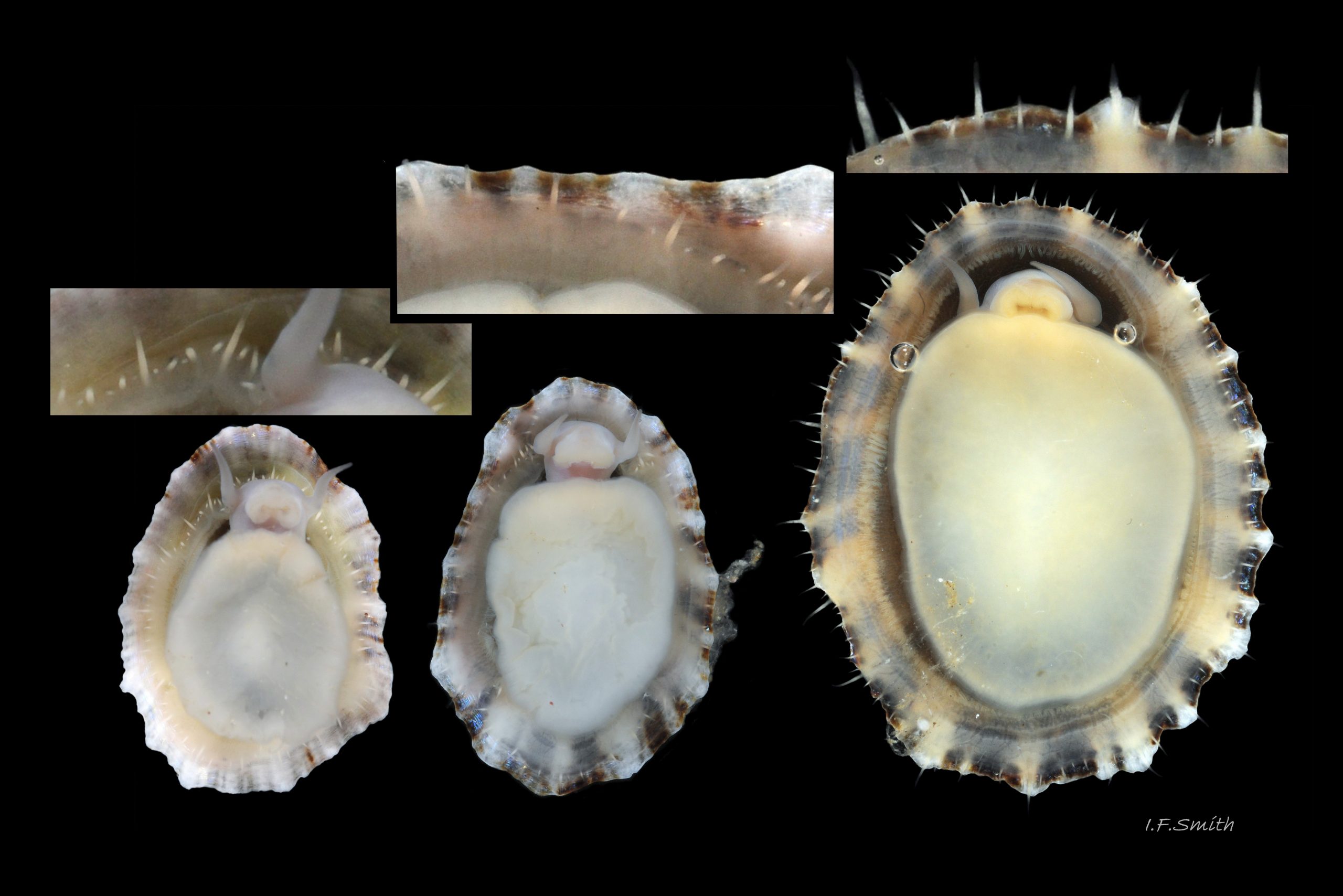
Spat, length 1mm, lack ridges on main anteroposterior axis and have broad, prominent, mid-lateral pigment lines running straight (not swept forwards or backwards) from apex to lip.
Body description
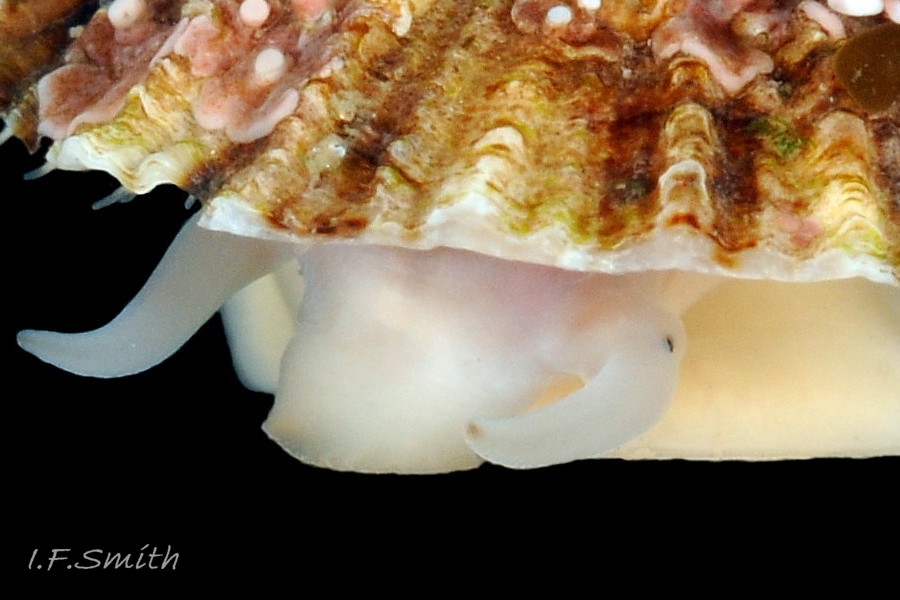
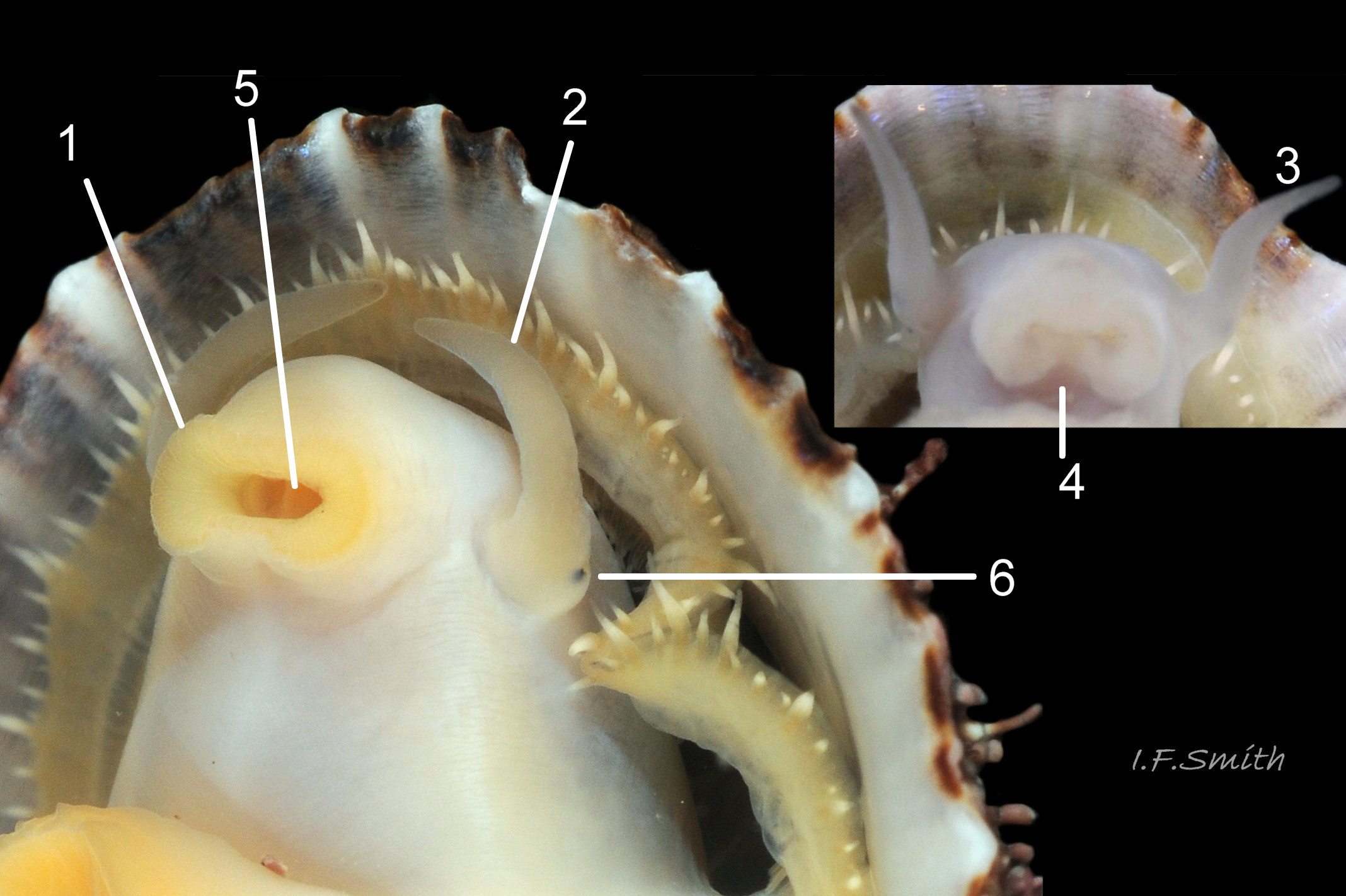
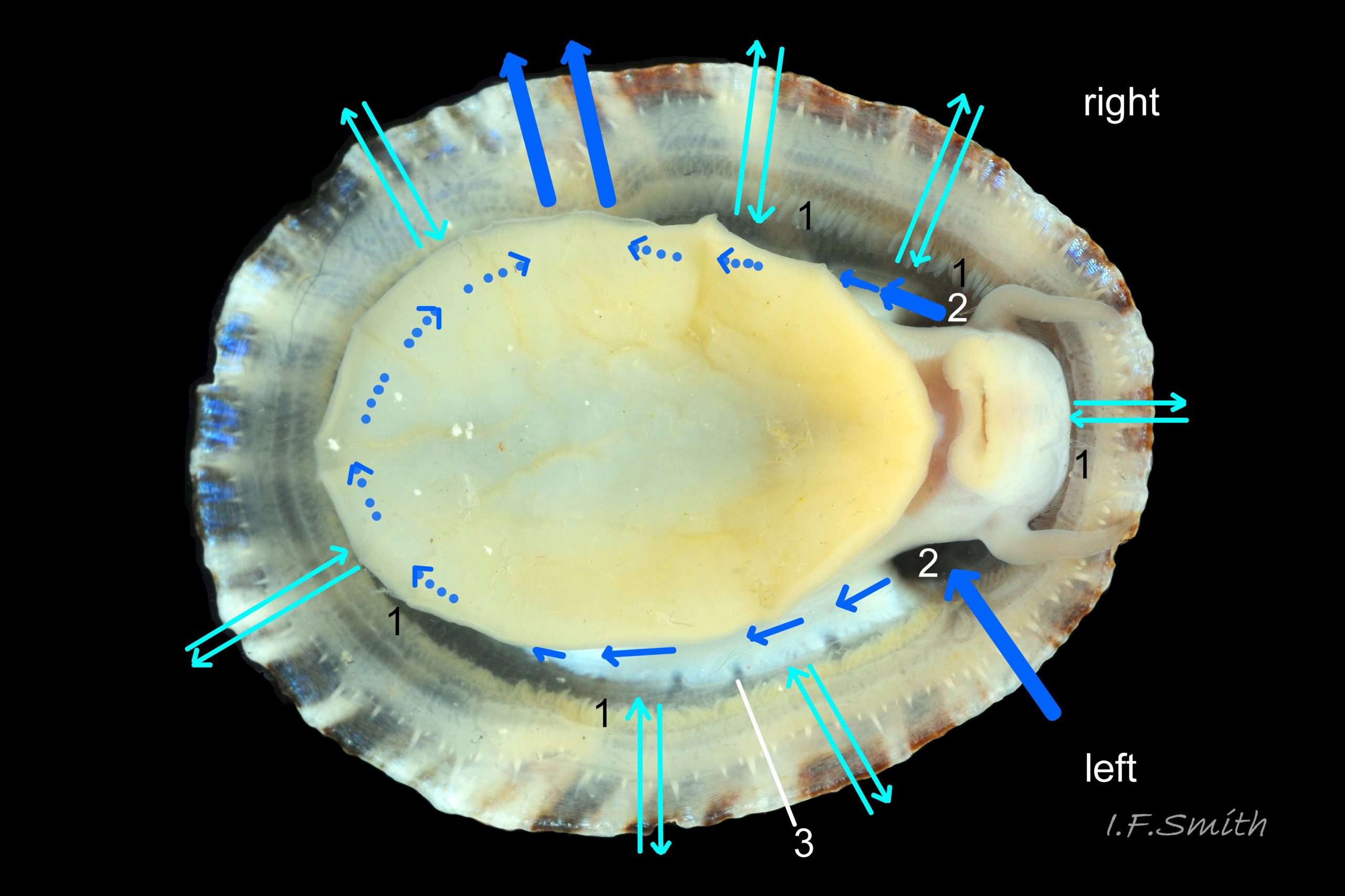
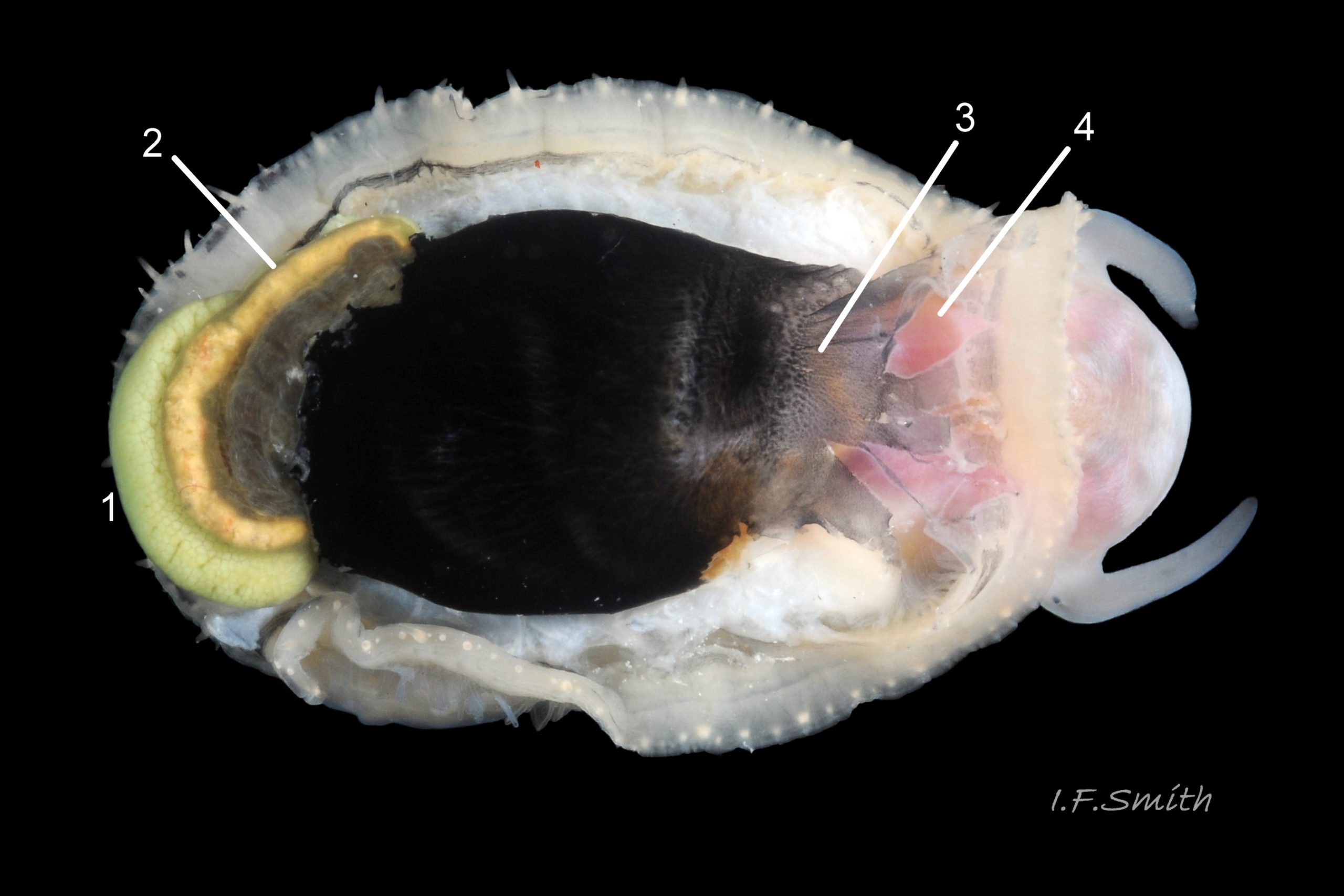
Translucent white head, with pink internal odontophore showing through 17 Patella ulyssiponensis; has substantial snout , folds in at posterior, with large mouth (transverse when shut) fringed by thick, yellow, outer lips (whitish when small); inner lips darker yellow, open laterally for protrusion of radula 18 Patella ulyssiponensis. Cephalic tentacles similar colour to head and/or mantle-skirt 19 Patella ulyssiponensis & 18 Patella ulyssiponensis , with small black eye in slight swelling at base. Eye is primitive (or degenerate) cavity, open to seawater and lined with black retina cells 17 Patella ulyssiponensis & 18 Patella ulyssiponensis. Mantle skirt translucent, usually darker than body colour; buff-white/cream 05 Patella ulyssiponensis, buff-grey 20 Patella ulyssiponensis, or yellow 21 Patella ulyssiponensis; colour most saturated when skirt retracted from shell-periphery 22 Patella ulyssiponensis . Skirt contains efferent pallial vessel 23 Patella ulyssiponensis. Mantle cavity consists of nuchal cavity over head, and pallial groove filled with pallial gills around entire periphery of foot-head 24 Patella ulyssiponensis; no ctenidium. Each gill is tongue-shaped leaflet attached by stalk to distal wall of pallial-groove and has densely ciliated groove on stalk and thickened rim 2 25 Patella ulyssiponensis.(Fretter & Graham, 1994). Mantle-edge has many white, off-white , cream or, on large specimens, yellow or orange pallial tentacles 26 Patella ulyssiponensis; basal half opaque becoming translucent and less intensely coloured distally; opaque basal parts distinct from translucent mantle-skirt that they arise from. Length of pallial tentacles alternates around perimeter with two or three short ones between each pair of long ones; length varies with extension 27 Patella ulyssiponensis. & 28 Patella ulyssiponensis. Visibility of pallial tentacles, and their position relative to shell, vary with degree of extension of mantle skirt 10 Patella ulyssiponensis. Pedal-retractor muscle, a U of white muscle bundles 28 Patella ulyssiponensis demarcated by gaps 24 Patella ulyssiponensis, attaches body/foot to shell 22 Patella ulyssiponensis. Sole of foot approximately circular with flattened anterior 29 Patella ulyssiponensis to broadly elliptical 05 Patella ulyssiponensis. Colour of sole varies: small whitish ones may have shadow of dark viscera if gonads undeveloped 30 Patella ulyssiponensis; adults, pale-yellow/cream, yellow 31 Patella ulyssiponensis or orange 21 Patella ulyssiponensis ; sometimes slightly-greenish tinted median zone where foot thinnest if greenish female gonads resting on inner surface of foot. White or yellowish-white 32 Patella ulyssiponensis sides of foot lack features such as epipodial tentacles. When crawling, usually only extended pallial tentacles and, perhaps, tips of cephalic tentacles protrude beyond shelter of shell. No penis as fertilization external.
Further detail visible with simple dissection
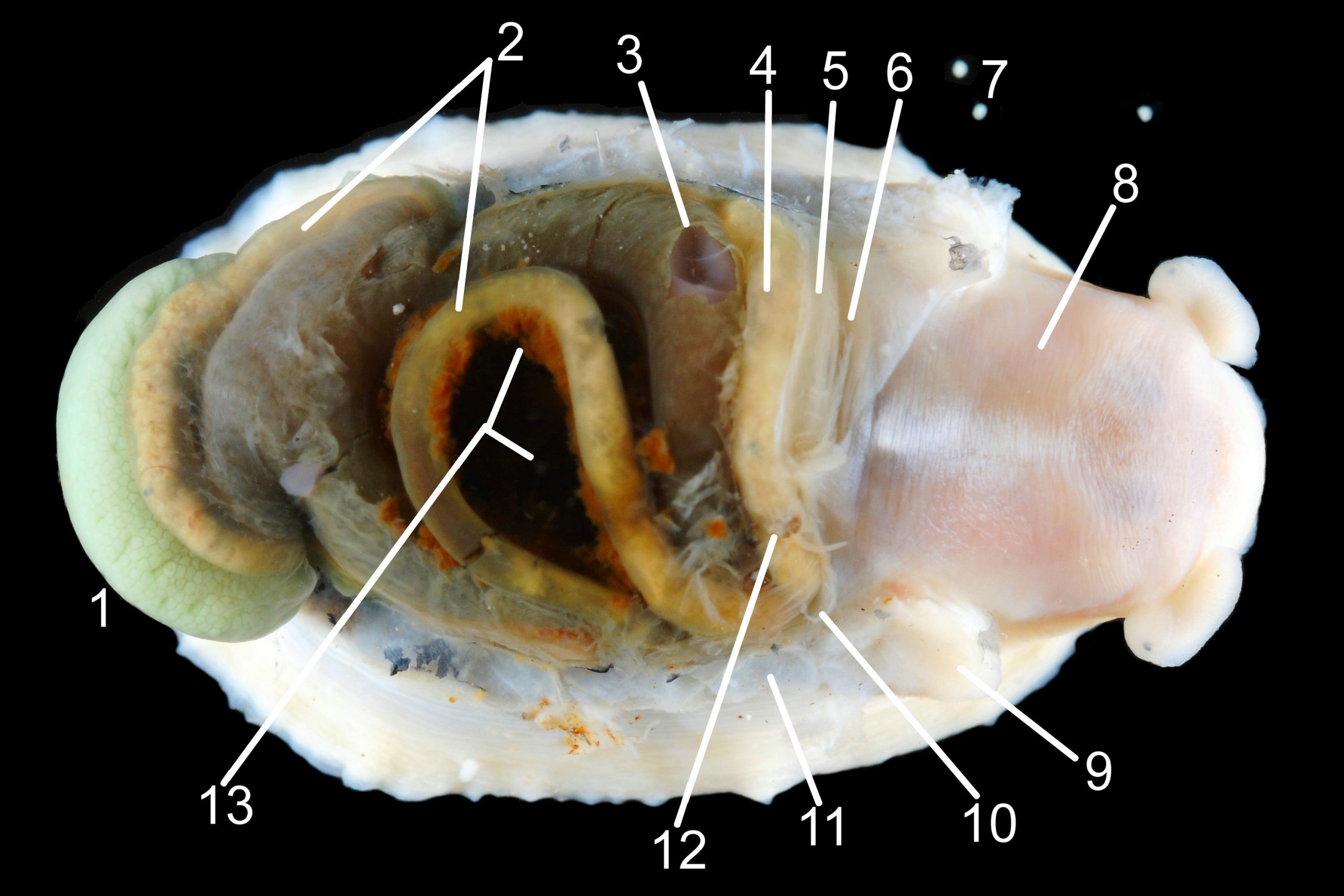
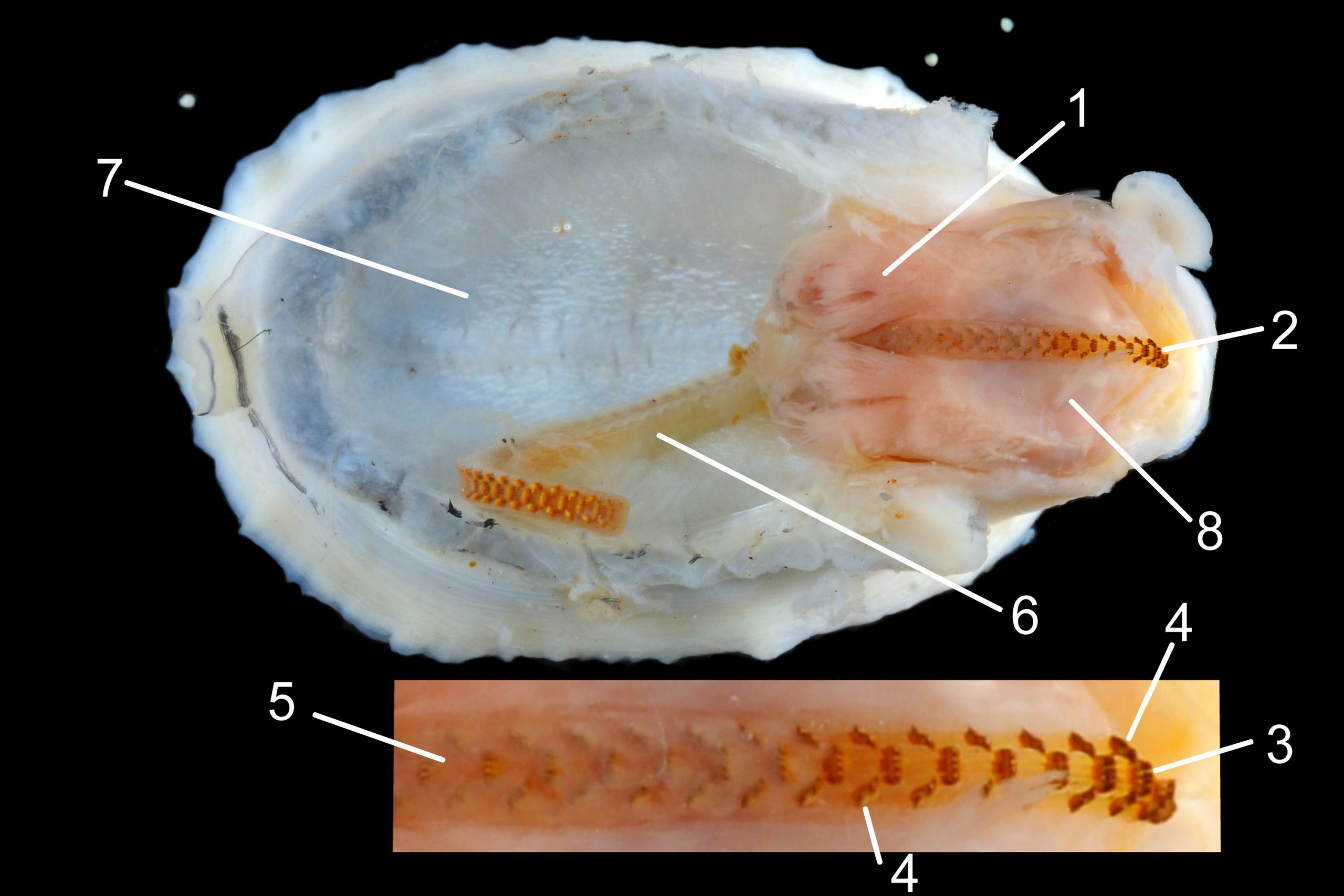
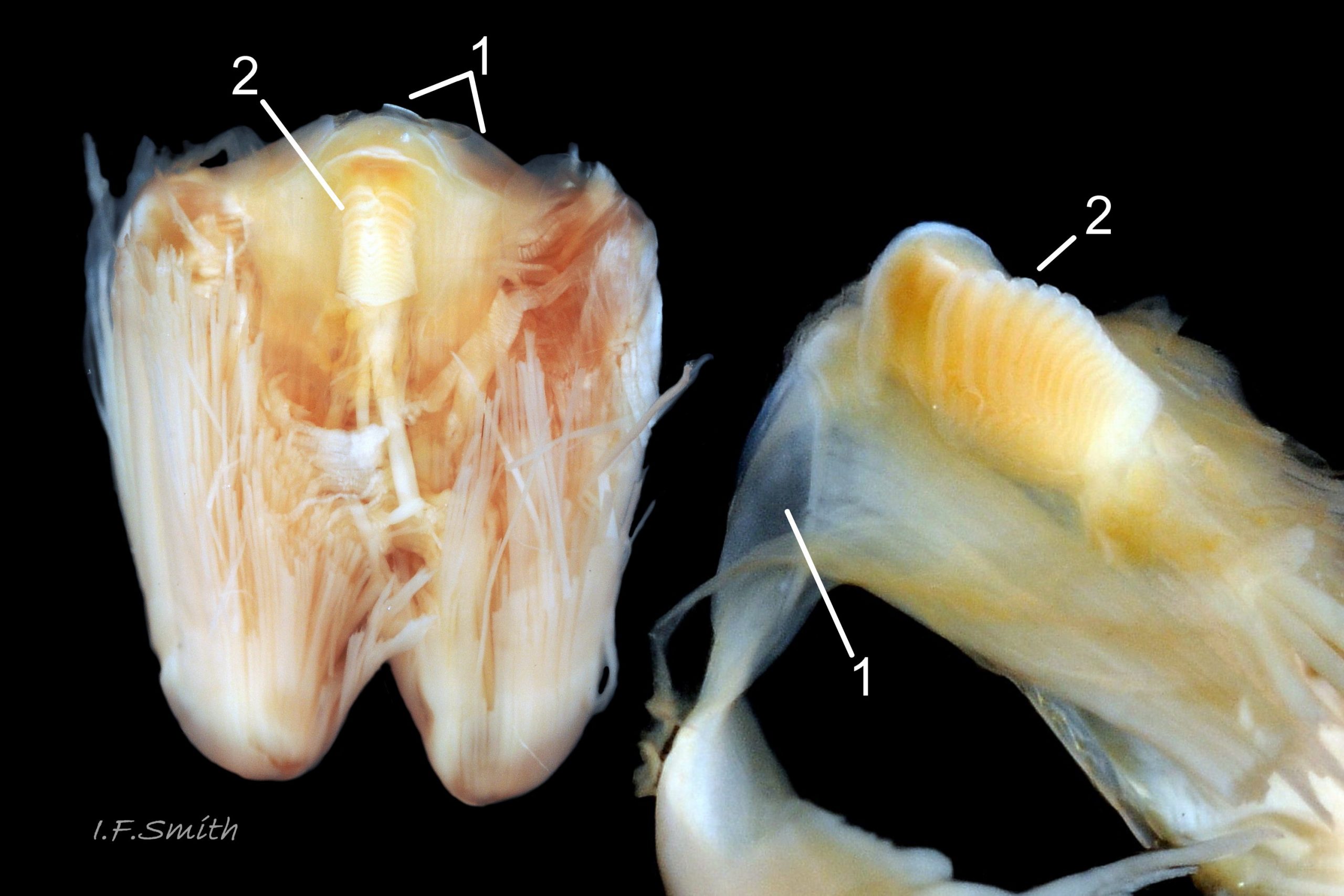
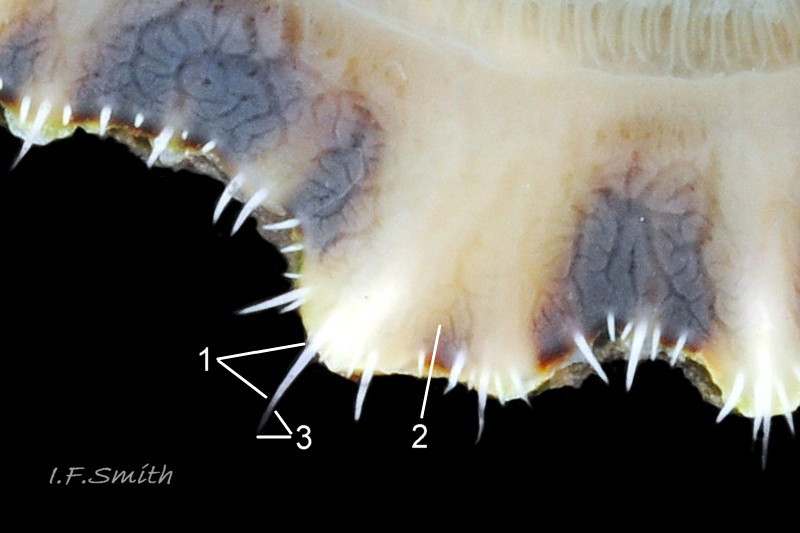
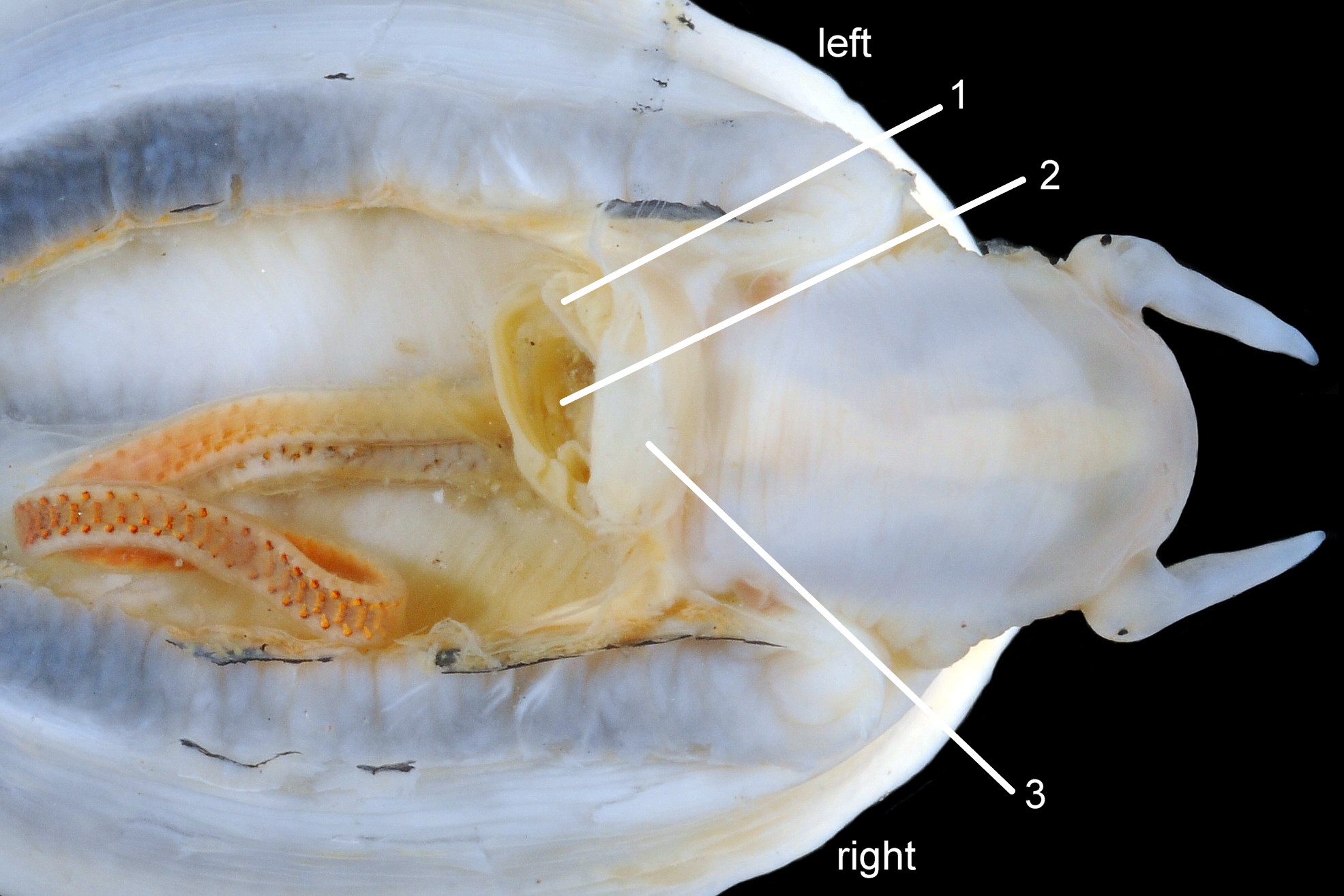
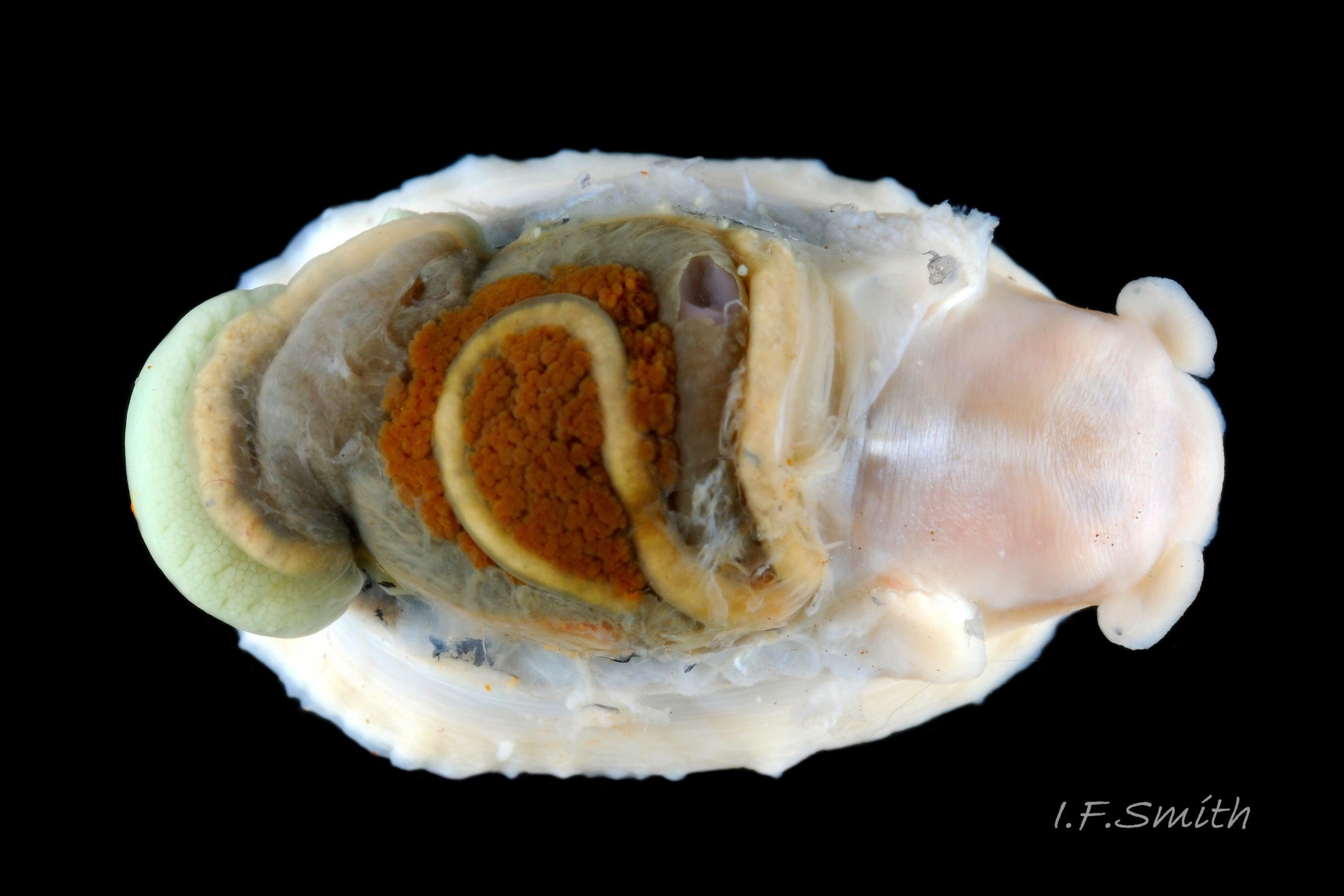
Shell removal by severing pedal-retractor muscle shows muscle-bundles clearly 28 Patella ulyssiponensis; anterior bundle on each side is largest and strongest as must firmly pull down shell further to anterior where bundles are absent. Shell removal exposes entire mantle 33 Patella ulyssiponensis subdivided into a) pale translucent mantle-skirt, b) narrow black band over pallial-groove containing gills, c) large black amphora-shaped area, sometimes paler near vertex, over viscera and nuchal cavity containing the head 34 Patella ulyssiponensis and d) pale anterior mantle-attachment. Removal of black amphora area of mantle reveals viscera 35 Patella ulyssiponensis including heart, digestive gland, intestine and, if in breeding condition, gonads protruding from below. When roof of nuchal cavity removed, translucent white head, usually showing pink of internal odontophore, is visible 35 Patella ulyssiponensis, and removal of viscera reveals radula, folded to fit in body 36 Patella ulyssiponensis. Removal of head’s epithelium reveals odontophore and anterior of radula 37 Patella ulyssiponensis. Radula long, relative to shell length, ( R/S 80-140%); shorter on average (but with overlapping range) than those of P. vulgata (113-230%) and P. depressa (140-270%) (Fretter & Graham, 1962, p.172). Fully mineralized, rust-coloured radular teeth, ready for action, clearly visible on hyaline shield at anterior; those further back partly obscured as in white radular sac. At anterior of radula, a white chitinous unarticulated jaw#, and white, cuticularized, triangular licker divided into plate-like ridges by deep transverse grooves 38 Patella ulyssiponensis. Each row of teeth arranged in docoglossan formula, 3+D+2+R+2+D+3: at centre, two pairs of large, unicuspid, pigmented lateral teeth (with small, unpigmented rachidian/median tooth hidden from easy view at their base), and near each margin of ribbon a single, tricuspid, pigmented, dominant-marginal tooth with, close-by, three inconspicuous, unicuspid, marginal teeth. Before and during summer breeding season, large gonads occur in mature adults between viscera and foot; female ovaries granular and yellow to green; male testes pink/orange with numerous interconnected tubules 39 Patella ulyssiponensis. When fully developed, gonads spread up around periphery of visceral mass 35 Patella ulyssiponensis.
Identification of British patellid limpets.
Exterior shell cannot be relied on, and shell-interiors can be confusing. Examination, in good light under magnification, of extended pallial tentacles on living animals is essential for consistent accurate discrimination of the three rock-dwelling Patella species. Best achieved with specimen adhering to underside of supported glass-sheet in black-based container of seawater.
Some morphologically intermediate forms can only be reliably identified by sequencing DNA or allozyme study. Intermediates result from similar environmental factors affecting different species in similar ways and are not hybrids (Sanna et al., 2011 and Sá-Pinto et al., 2007). For the purpose of recording for distribution schemes it is advisable to disregard intermediates unless DNA or allozymes can be employed, especially beyond or on the limits of known distributions. Intermediates most frequent near limit of distributions of P. depressa and P. ulyssiponensis in Isle of Wight , perhaps because conditions not optimal (Fretter and Graham, 1994).
Key identification features of typical British specimens.
Patella ulyssiponensis
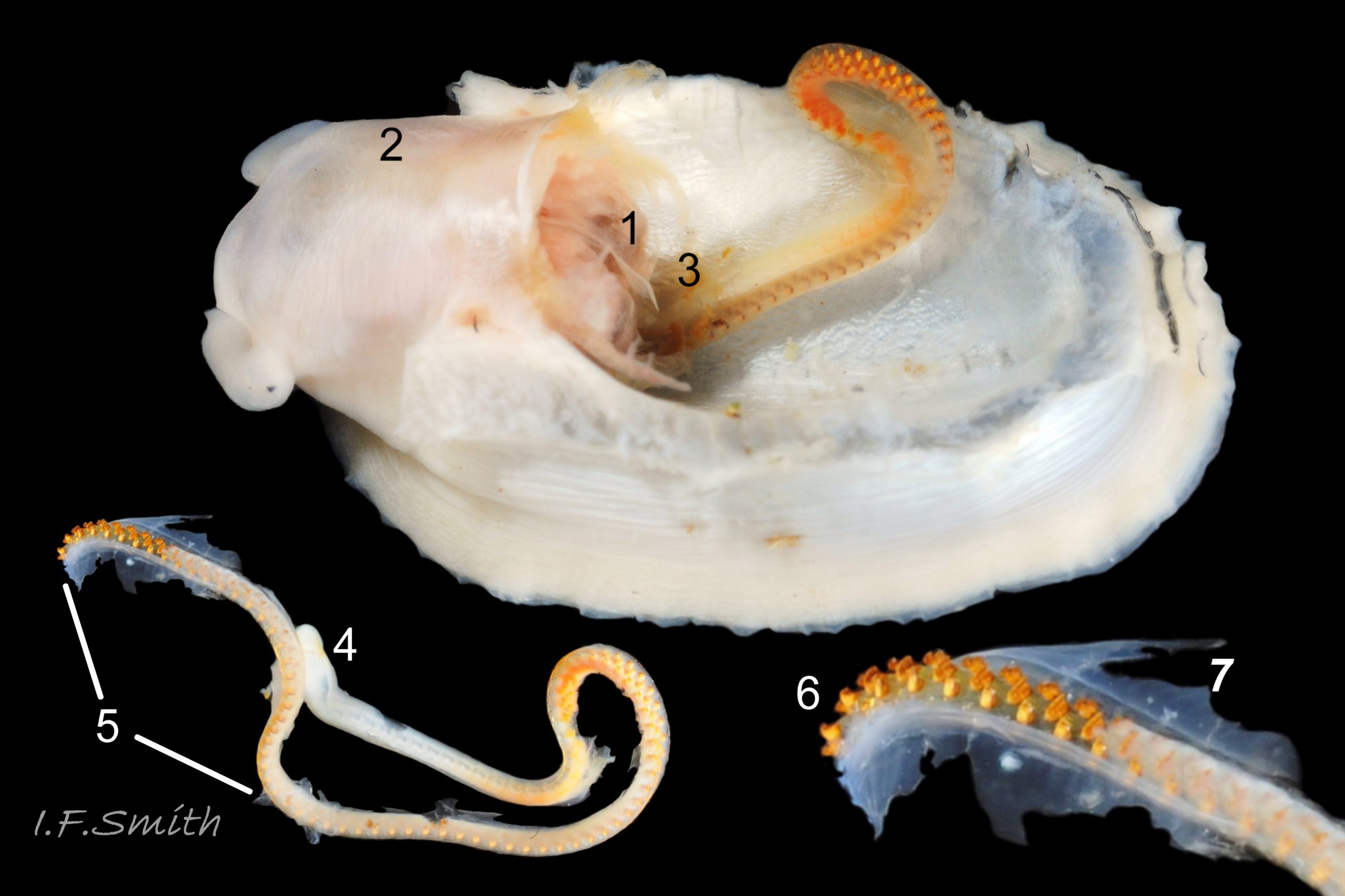
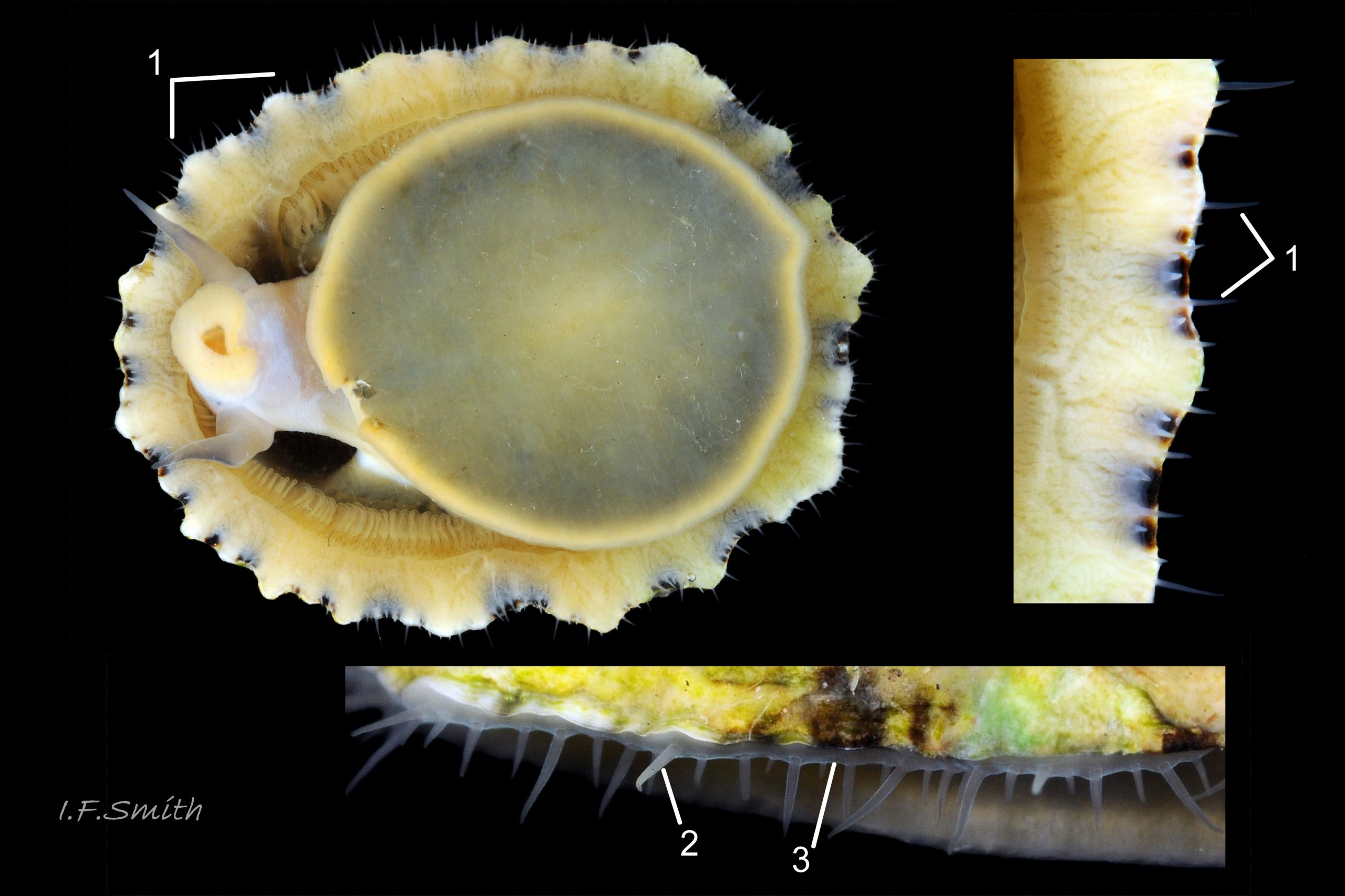
1. Basal half of pallial tentacles has opaque pigment which can be white, off-white, cream or, on large specimens, yellowish or orange. The distal half fades to a translucent tip. Opaque basal half is often distinct from translucent mantle-skirt that they arise from, so it is possible to confuse with P. depressa. It is important to use pallial tentacles in combination with foot-colour/shell-length for identification. Examples at 26 Patella ulyssiponensis.
2. Foot that is NOT pitch-brown/black or dark khaki. It can be whitish when young 30 Patella ulyssiponensis becoming yellowish 31 Patella ulyssiponensis and, sometimes, orange with age 21 Patella ulyssiponensis. Juveniles under 12mm length may show a blackish internal shadow through the thin pale translucent foot 30 Patella ulyssiponensis as they lack gonads above the foot that mask the dark viscera in adults.
Similar species
Patella vulgata
Extremely variable species; foot colours and nearly all shell-features have overlaps with P. depressa and P. ulyssiponensis.
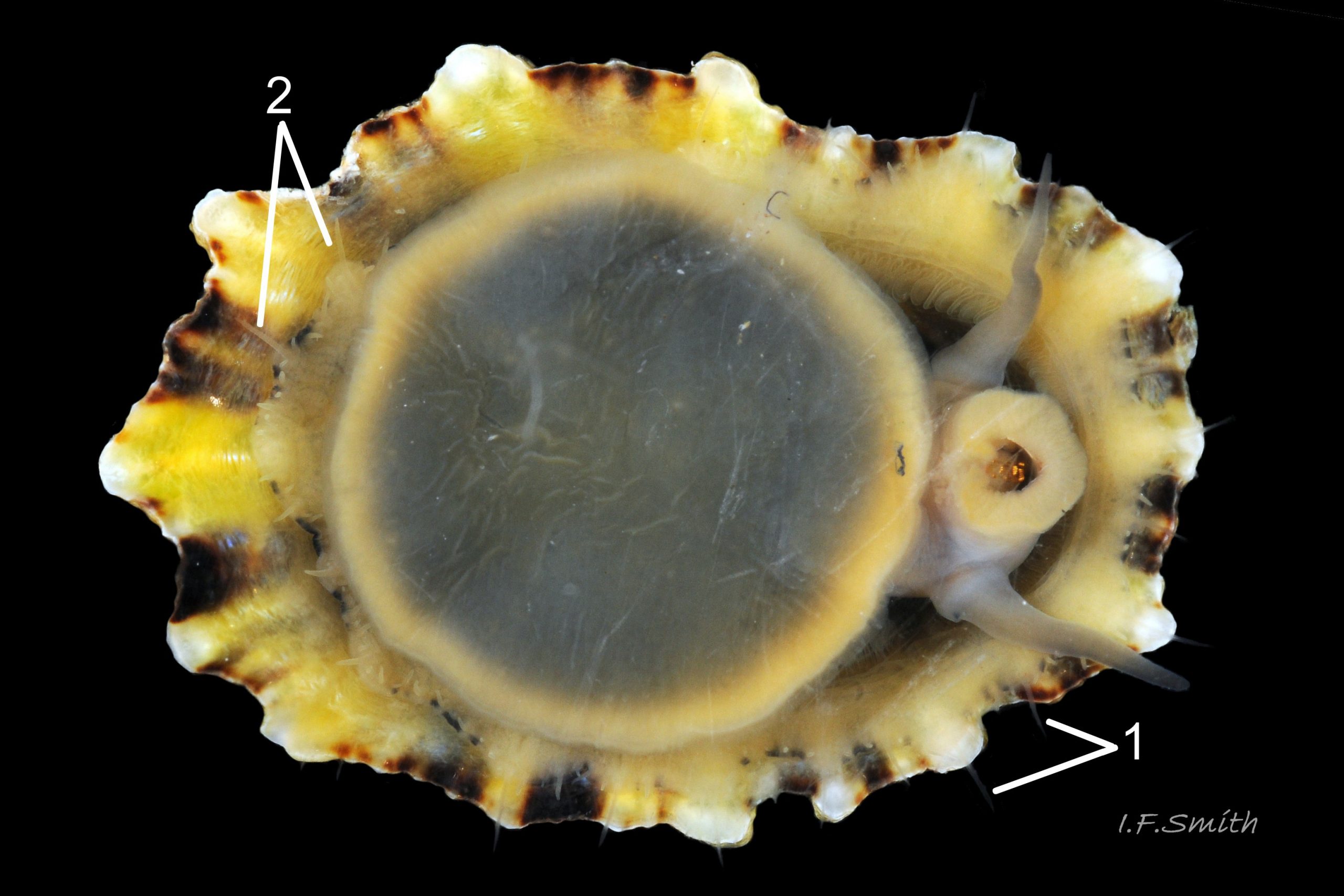
1. Pigment-less pallial tentacles are slender, translucent and same colour as mantle-skirt they arise from. 40 Patella ulyssiponensis.
Cautions:
Pallial tentacles of P. vulgata may look white when arise from colourless mantle-skirt in some lighting, but no pigment 41 Patella ulyssiponensis.
Translucency and fineness of pallial tentacles of P. vulgata often make discernment difficult, especially when mantle skirt retracted from shell-rim and pallial tentacles viewed against shell 42 Patella ulyssiponensis; often virtually invisible when out of water as may be retracted as well as highly translucent 43 Patella ulyssiponensis.
Foot colour of P. vulgata varies greatly, sometimes orange resembling P. ulyssiponensis 53 Patella ulyssiponensis.
Shell interior can be white or tinted orange in P. vulgata 53 Patella ulyssiponensis and 44 Patella ulyssiponensis.
Patella depressa
[1 & 2 in combination, not singly, are diagnostic of typical specimens but exclude intermediates.]
1. Pigmented pallial tentacles are opaque chalky-white for more than half of extended-length; may have translucent tip; distinctly whiter than buff mantle-skirt from which they arise 45 Patella ulyssiponensis. Even when mantle-skirt retracted, pallial tentacles often clearly visible contrasting with the darker mantle 46 Patella ulyssiponensis album.
2. Sole of foot pitch-brown 47 Patella ulyssiponensis to black 46 Patella ulyssiponensis.
3. On shell-interior, whitish projecting points of ribs have short, unglazed, chalky, pure-white central line, but reduced or lacking where projecting points of ribs eroded 48 Patella ulyssiponensis. [This feature recently recognised by S. Payne, and applies to all in large sample examined by IFS. Unsure yet if universal on P. depressa and exclusive of P. vulgata and P. ulyssiponensis.]
Caution:
Shell interior can be orange-cream in P. depressa 49 Patella ulyssiponensis
Patella caerulea Linnaeus, 1758.
Does not occur in Britain. In Iberia and Mediterranean, separation from it of some specimens of P. ulyssiponensis could not be achieved with foot colour and shell morphology by Sanna et al. (2011) who relied on the use of DNA sequencing. They did not mention attempting the use of pallial tentacle colour on live specimens; it may be worth investigation. See Sanna et al. for images of P. caerulea.
Habits and ecology
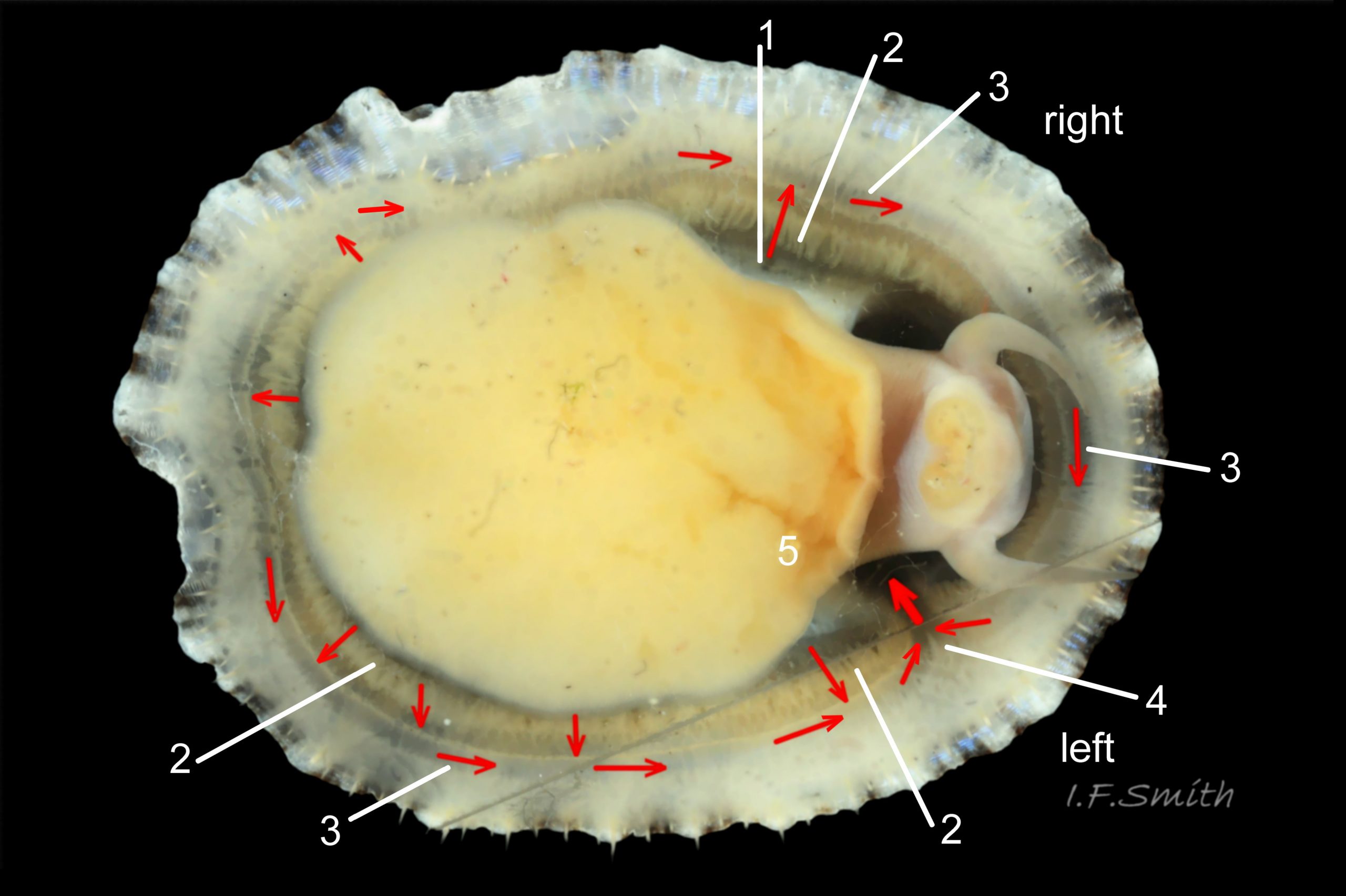
P. ulyssiponensis is a southern species which reaches its northern limit in south-west Norway and locally at cold winter-sea areas of Baltic, North Sea and north-east Irish Sea. It lives on rocky shores with Ballantine (1961) wave exposure scale 1- 4 or 5 where turbidity does not prevent algal growth. It is often the dominant species of limpet at mid- and low-tide levels on extremely and very exposed shores (scales 1 & 2). It can be common on exposed (scale 3) lower shores, and present on semi-exposed shores (scale 4) but largely confined to lower levels or to pools lined with encrusting calcareous algae at higher shore levels. On fairly sheltered (scale 5) shores it is absent or confined to pools. It is usually found on bedrock, not on shingle or loose boulders, and it extends into the sublittoral zone. It is unable to produce very large, thick, high-domed shells, like those of some P. vulgata 50 Patella ulyssiponensis, to resist desiccation on drained rock on upper shores. P. ulyssiponensis is reported to be a consistently homing species (Branch, 1981a); adults always after feeding-excursions seeking to return to same position where a deep home-scar can be developed when substrate is relatively soft encrusting calcareous algae 07 Patella ulyssiponensis. Differences in amount of opaque, white, porcelaneous material on interior of shells at different localities may be due differences in suitability of environment 15 Patella ulyssiponensis. Locomotion by retrograde waves alternating on each side (ditaxic) of sole; muscles alternately compress/relax against blood trapped between them to create waves. Feeding: most frequently grazes on calcareous encrusting algae and Corallina, ingesting the algae and organic deposits on their surface. Grazing is facilitated by powerful muscles in large buccal mass, and by rust-coloured iron-reinforced teeth on long radula with plentiful replacements for worn teeth 36 Patella ulyssiponensis Length varies seasonally; shorter when wear of active feeding exceeds growth rate. Patella spp. wear out up to two rows of teeth per day (Sigel, 2008 ). About four rows of teeth are in contact with substrate during feeding; loose particles are retained by rim of surrounding jaw# and the licker 38 Patella ulyssiponensis which sweeps them up at the end of the radula stroke. Long coiled intestine compacts faeces (often yellowish from high lime content obtained from calcareous algae) 35 Patella ulyssiponensis into firm faecal strings that will not contaminate gills in pallial groove; compensates for adults lacking hypobranchial gland to produce mucus to bind particles exiting from nuchal cavity. Cilia on roof of nuchal cavity and side of foot conduct faecal matter from anus in nuchal cavity to middle of right side 24 Patella ulyssiponensis. Faeces and debris accumulate there until periodic sharp contraction of pedal-retractor muscle clamps shell down and forcefully flushes water and waste out of shell (Fretter & Graham 1962 & 1994). When limpets removed from rock, accumulated pile of faecal strings often found in position. Cilia also create inhalent water-current from left of head through nuchal cavity, where urogenital openings located, and thence carry excreta and ova/sperm to exterior. Colourless interior shell-layers may be stained orange by digestive gland when feeding on red algae in both P. ulyssiponensis 04 Patella ulyssiponensis and P. vulgata 43 Patella ulyssiponensis.Predators reported to be able to dislodge P. vulgata shells probably take P. ulyssiponensis too; they include gulls (Larus spp.), oystercatchers (Haematopus ostralegus), crabs, starfish and rats. Nucella lapillus bores through the shell, usually to the pedal-retractor muscle where the adjacent viscera are accessible obliquely to its radula without having to bore through the thick amphora# shell-layers covering the viscera 51 Patella ulyssiponensis. Boring takes several days, but is rewarded with a large food supply, providing the Nucella isn’t dislodged before completion . Respiration: gill-cilia create gentle local inhalent respiratory water currents all around perimeter of animal from adjacent shell-rim onto gills, and exhalent currents below gills back to shell-rim 24 Patella ulyssiponensis(Yonge & Thompson, 1976). Densely ciliated groove on stalk and rim of each gill-lamella catches and removes large particles of detritus that would clog gill (Fretter & Graham, 1994) 2 25 Patella ulyssiponensis. Blood passes from viscera and foot via vessels through gaps in encircling pedal-retractor muscle 23 Patella ulyssiponensis into gills for oxygenation, and thence into encircling efferent pallial vessel in mantle-skirt, which carries blood to left of nuchal cavity and through its roof to elongated heart behind left of cavity 52 Patella ulyssiponensis for recirculation to head, foot and viscera (Fretter & Graham, 1994). On shells with thick porcellaneous interior layers, efferent pallial-vessel leaves a mark where it passes through gap in gills to enter nuchal cavity 01 Patella ulyssiponensis & 14 Patella ulyssiponensis. Breeding season varies geographically; June-September N.E. England, June-November S.W. England, and precise timing varies year to year (Fretter & Graham, 1994). External fertilization, so close proximity of sexes required for success. Sperm and ova shed into water column, ova individually. Eggs hatch as free trochophore larvae (stage passed within egg by most “less-primitive” spp.) in plankton before transforming to veligers and, after a short planktonic-life, settling on lower shore and assuming limpet form. Spat, when 1mm long, have eight radiating ridges; P. vulgata has five ridges on right, four on left. P. depressa has ten (Fretter & Graham, 1994). Some move to mid-tide level when shell-length 5mm.
Distribution and status
Mediterranean, Black Sea and North-east Atlantic from Morocco to Shetland and Bergen, Norway (Høisæter, 2009). Not in the colder waters of a) the Baltic b) North Sea from Stavanger to Le Havre and from Flamborough to Beachy Head c) north-east of Irish Sea from Kircudbright or Dumfries to Anglesey.
GBIF map www.gbif.org/species/5190390 ; Belgian and Dutch records are from flotsam (Fretter & Graham, 1994, p.464) and records on Macaronesian islands are misidentified P. aspera Röding, 1798 (“note” at www.marinespecies.org/aphia.php?p=taxdetails&id=456570)
U.K. distribution map NBN species.nbnatlas.org/species/NHMSYS0021056398
Key identification features of typical British specimens.
Patella ulyssiponensis
1) Basal half of pallial tentacles has opaque pigment which can be white, off-white, cream or, on large specimens, yellowish or orange. The distal half fades to a translucent tip. Opaque basal half is often distinct from translucent mantle-skirt that they arise from, so it is possible to confuse with P. depressa. It is important to use pallial tentacles in combination with foot-colour/shell-length for identification. Examples at 26 Patella ulyssiponensis.
2) Foot is NOT pitch-brown/black or dark khaki. It can be whitish when young 30 Patella ulyssiponensis becoming yellowish 31 Patella ulyssiponensis and, sometimes, orange with age 21 Patella ulyssiponensis. Juveniles under 12mm length may show a blackish internal shadow through the thin pale translucent foot 30 Patella ulyssiponensis as they lack gonads above the foot that mask the dark viscera of adults.
Similar species
Patella vulgata
Extremely variable species; foot colours and nearly all shell-features have overlaps with P. depressa and P. ulyssiponensis.
1. Pigment-less pallial tentacles are slender, translucent and same colour as mantle-skirt they arise from. 40 Patella ulyssiponensis.
Cautions:
Pallial tentacles of P. vulgata may look white when arise from colourless mantle-skirt in some lighting, but no pigment 41 Patella ulyssiponensis.
Translucency and fineness of pallial tentacles of P. vulgata often make discernment difficult, especially when mantle skirt retracted from shell-rim and pallial tentacles viewed against shell 42 Patella ulyssiponensis; often virtually invisible when out of water as may be retracted as well as highly translucent 43 Patella ulyssiponensis.
Foot colour of P. vulgata varies greatly, sometimes orange resembling P. ulyssiponensis albumPvhigh.
Shell interior can be white or tinted orange in P. vulgata albumPvhigh and 44 Patella ulyssiponensis.
Patella depressa
[1 & 2 in combination, not singly, are diagnostic of typical specimens but exclude intermediates.]
1. Pigmented pallial tentacles are opaque chalky-white for more than half of extended-length; may have translucent tip; distinctly whiter than buff mantle-skirt from which they arise 45 Patella ulyssiponensis. Even when mantle-skirt retracted, pallial tentacles often clearly visible contrasting with the darker mantle 46 Patella ulyssiponensis.
2. Sole of foot pitch-brown 47 Patella ulyssiponensis to black 46 Patella ulyssiponensis.
3. On shell-interior, whitish projecting points of ribs have short, unglazed, chalky, pure-white central line, but reduced or lacking where projecting points of ribs eroded 48 Patella ulyssiponensis. [This feature recently recognised by S. Payne, and applies to all in large sample examined by IFS. Unsure yet if universal on P. depressa and exclusive of P. vulgata and P. ulyssiponensis.]
Caution:
Shell interior can be orange-cream in P. depressa 49 Patella ulyssiponensis
Patella caerulea Linnaeus, 1758.
Does not occur in Britain. In Iberia and Mediterranean, separation from it of some specimens of P. ulyssiponensis could not be achieved with foot colour and shell morphology by Sanna et al. (2011) who relied on the use of DNA sequencing. They did not mention attempting the use of pallial tentacle colour on live specimens; it may be worth investigation. See Sanna et al. for images of P. caerulea.
Acknowledgements
I gratefully acknowledge Dr Sebastian Payne for information, discussion and help during shore-work. Any errors or omissions are the responsibility of the author.
Links and references
Akşit, D. & Falakil Mutaf, B. 2011. The external morphology of the gill of Patella caerulea L. (Mollusca: Gastropoda). Turk. J. Zool. 35(4) 603-606. Tübitak. Turkey. PDF contains SEM images of gill.
www.google.co.uk/search?q=patella+gill+ciliated+groove&am…
Backeljau, T. 1986. Lijst van de recente mariene mollusken van Belgie Koninklijk Belgisch Instituut voor Natuurwetenschappen, Brussels.
PDF at www.marinespecies.org/imis.php?module=ref&refid=4414
Barber, A.H., Lu, D. & Pugno, N.M. 2015 Extreme strength observed in limpet teeth The Royal Society.
rsif.royalsocietypublishing.org/content/12/105/20141326
Branch, G.M. 1981a. The biology of limpets. Oceangr. Mar. Biol. Ann. Rev. learning.watfordboys.org/mod/resource/view.php?id=4730
Branch, G.M. 1981b. The biology of limpets. Oceangr. Mar. Biol. Ann. Rev. www.google.co.uk/search?q=Patella+vulgata+blood+circulati...
Cohen, A.L. & Branch, G.M. 1992. Environmentally controlled variation in the structure and mineralogy of Patella granularis shells from the coast of southern Africa: implications for palaeotemperature assessments. Palaeogeography, palaeoclimatology, palaeoecology, 91: 49-57. www.whoi.edu/fileserver.do?id=163844&pt=2&p=36767
Forbes, E. & Hanley S. 1849-53. A history of the British mollusca and their shells. vol. 2 (1849), London, van Voorst. (As Patella athletica; PDF at archive.org/stream/historyofbritish02forb#page/424/mode/2up Use slide at base of page to select pp.425-429.)
Fretter, V. and Graham, A. 1962. British prosobranch molluscs. London, Ray Society.
Fretter, V. and Graham, A. 1994. British prosobranch molluscs. Revised and updated edition. London, Ray Society.
Gmelin, J.F. (1791) Vermes. In Gmelin J.F. (Ed.) Caroli a Linnaei Systema Naturae per Regna Tria Naturae, Editio Decima Tertia, Aucta Reformata. Tome 1, Pars 6 (Vermes). G.E. Beer, Lipsiae [Leipzig], pp. 3021-3910., available online at www.biodiversitylibrary.org/item/83098#5 Original description on p.692 of PDF .
Goshima, S., Ilano, A.S., Ito, A. & Nakao, S. 2002. Seasonal and tidal-height variations in body weight and radular length in Nodilittorina radiata (Eydoux & Souleyet, 1852). J. Mollus. Stud. 68: 197-203.
PDF at mollus.oxfordjournals.org/content/68/3/197.full.pdf+html
Graham, A. 1988. Prosobranch and pyramidellid gastropods. London.
Høisæter, T. 2009. Distribution of marine, benthic, shell bearing gastropods along the Norwegian coast. Fauna Norvegica 28: 5-106.
pdf at www.ntnu.no/ojs/index.php/fauna_norvegica/article/view/563
Jeffreys, J.G. 1862-69. British conchology. vol. 3 (1865). London, van Voorst. (As Patella vulgata var. 4 depressa, incorrectly attributed to Pennant.); Free PDF at archive.org/stream/britishconcholog03jeff#page/236/mode/2up . Use slide at base of page to select pp.237.
MacClintock, C. 1967. Shell structure of patelloid and bellerophontid gastropods (Mollusca). Peabody Museum of Natural History, Yale University. Bulletin 22. pdf at
www.google.co.uk/?gws_rd=ssl#q=MacClintock%2C+C.+1967.+Sh….
215 pages, may take a few minutes to download. Contents on page v.(= p.6 of pdf). To find pages on pdf add 1 to Roman numerals, and add 11 to modern numerals.
Pennant, T. (1777). British Zoology, vol. IV. Crustacea. Mollusca. Testacea. London. i-viii, 1-154, pls. 1-93.,
Page 142 biodiversitylibrary.org/item/127011#page/168/mode/1up
Pl. 89 46. biodiversitylibrary.org/item/127011#page/361/mode/1up
Sanna, D., Dedola, G. L., Lai, T., Curini-Galletti, M. & Casu, M. 2011. PCR-RFLP: A practical method for the identification of specimens of Patella ulyssiponensis s.l. (Gastropoda: Patellidae), Italian Journal of Zoology,
pdf at www.researchgate.net/publication/233126771_PCR-RFLP_A_pra…
Sá-Pinto, A., Branco,M., Harris, D.J. & Alexandrino, P. 2005. Phylogeny and phylogeography of the genus Patella based on mitochondrial DNA sequence data. J. Exp. Mar. Biol. Ecol. 325: 95-110.
Sá-Pinto, A., Alexandrino, P. & Branco,M. 2007. High genetic differentiation with no evidence of hybridization between four limpet species (Patella spp.) revealed by allozyme loci. Scientia Marina 71(4): 801-810. Barcelona. pdf at www.vliz.be/imisdocs/publications/131981.pdf
Yonge, C.M. and Thompson, T.E. 1976. Living marine molluscs. London.
Current taxonomy: World Register of Marine Species (WoRMS)
www.marinespecies.org/aphia.php?p=taxdetails&id=140684
GLOSSARY
amphora – (on interior of limpet shell) Roman amphora-shaped area enclosed by scars of pedal-retractor muscle and anterior mantle-attachment.
anteroposterior – (of linear feature) aligned from anterior to posterior.
aperture – mouth of gastropod shell; outlet for head and foot.
apex – earliest formed part of a gastropod shell, the summit of the cone. (In this limpet-account restricted to the exterior of the shell, and “vertex” used for the interior.)
auct. – (abbreviation of “auctorum” = “of authors”) name, often of another valid species, used in error for this one by other author(s). en.wikipedia.org/wiki/Auctorum
cephalic – (adj.) of or on the head.
cilia – (pl.) microscopic linear extensions of membrane that move in rhythmic waves to create locomotion, or move particles and liquids e.g. inhalent water currents. (“cilium” singular). (Electron scanning microscope image at 18 Lepidochitona cinerea )
ciliary – (adj.) relating to or involving cilia.
coll. – in the collection of (named person or institution) (compare with legit).
conoid – shaped like a cone.
ctenidium – comb-like molluscan gill; usually an axis with a row of filaments either side.
ELWS – extreme low water spring tide (usually near March and September equinoxes).
epipodial – (adj.) of the epipodium (collar or circlet running round sides of foot of some gastropods).
epithelium – membranous covering of internal and external surfaces of animal’s body, e.g. skin and lining of tubes and cavities.
head scar – term used by many British authors for patch of different shell-material, and often different colour, near vertex of interior of limpet shell; misnomer as the mobile head, free of any attachment to the shell or mantle-roof of the nuchal cavity cannot make a scar. A preferable term is “vertex patch”.
height – (of limpet) perpendicular distance from apex to plane of aperture-rim (best measured with callipers).
hyaline shield – transparent sheet of chitin at anterior of radula that rests on bolsters of odontophore; attachment point for retractor muscles of radula; helps guide food particles into mouth.
jaw – unarticulated chitinous structure that encloses inner lips of Patella spp. at sides and anterior.
legit – (abbreviation; leg.) collected/ found by (compare with coll.)
licker – cuticularized structure with plate-like ridges and deep transverse grooves at tip of radula of Patella spp.; retains and sweeps up food particles.
Macaronesia – Madeira, Canary Islands, Cape Verde Islands and Azores.
mantle – sheet of tissue covering visceral mass of molluscs. Secretes shell of shelled species, and forms part or all of dorsal body surface (notum) of those without shells. (See mantle skirt.)
mantle skirt – extension on gastropods of mantle proper as a flap roofing a cavity containing gills, genital and renal openings, anus etc. On limpets, skirt and cavity extend around periphery of animal.
MLWS – mean low water spring tide level (mean level reached by lowest low tides for a few days every fortnight; Laminaria or Coralline zone on rocky coasts).
nuchal – (adj.) of nape of the neck.
nuchal cavity – cavity roofed by mantle skirt that contains head of limpet; part of mantle cavity (remainder consists of pallial groove on each side of body).
ovoid – egg-shaped, (as a solid or in outline).
ovate – egg-shaped, (as a solid or in outline).
pallial groove band – shell material deposited on interior of shell by strip of black mantle roofing the pallial groove that contains the gills. On British Patella spp. it is often clouded-white.
pedal retractor muscle – strong muscle that retracts foot into shell of most gastropods, but on limpets is used to clamp shell to substrate, a.k.a. “foot muscle”.
porcellaneous – resembling vitreous glazed ceramic material.
retrograde – (of locomotion waves on foot) waves travel from anterior to posterior.
scar – mark on shell made by attachment point of muscle or other body part.
skirt shell layer – shell material deposited on interior of shell by mantle skirt. On British Patella spp. colourless when deposited, and clouded white, or transparent showing the colours of the outer layer. Crystalline structure causes short lines of blue iridescence parallel to the aperture rim on all four British species of Patella when the light is right.
tricuspid – (of tooth) having three points.
trochophore – spherical or pear-shaped larva that swims with aid of girdle of cilia. Stage preceding veliger, passed within gastropod egg in most spp. but free in plankton for patellid limpets, most Trochidae and Tricolia pullus.
unicuspid – (of tooth) having a single point.
veliger – shelled larva of marine gastropod or bivalve mollusc which swims by beating cilia of a velum (bilobed flap).
vertex – angle at highest point on interior of limpet-shell. [Synonym of “apex”, chosen (by IFS) to help avoid confusion with the highest point, apex, on the exterior. In classical Latin “vertex” was used for the “pole of the heavens”, obviously only seen from below.]
vertex patch –layer of different shell-material, and often different colour, at vertex of interior of limpet shell. (See “head scar”.)