Click image to enlarge with full caption. Main text below slider.
Authors: Ian F. Smith (text), Alen Petani, Simon Taylor and Inga Williamson. December 2018.
Key Identification Features & Similar Species under image 02 Rissoa membranacea
Synonyms:
Turbo membranaceus, J. Adams, 1800; Turboella cornea (Loven, 1846); Rissoa labiosa (Montagu, 1803); Rissostomia membranacea J. Adams, 1800; Rissoa membranacea var. cornea (Loven, 1846); Rissoa membranacea var. gracilis (Forbes & Hanley, 1850); Rissoa membranacea var. labiosa (Montagu, 1803); Rissoa membranacea var. minor Jeffreys, 1867; Rissoa membranacea var. octona auct. non Linnaeus, 1767.
Current taxonomy: World Register of Marine Species (WoRMS) http://www.marinespecies.org/aphia.php?p=taxdetails&id=141359
Meaning of name:
Rissoa = named for G.A. Risso.
membranacea = (Latin) like a membrane [refers to shell thickness of specimens first described].
Vernacular: Stor tangsnegl (Danish); Vliezig drijfhorentje (Dutch);
dünnschalige Rissoa (German); Större bandtångsnäcka (Swedish);
GLOSSARY below.
Introduction
R. membranacea is a very varied species with markedly different shells in different environments that have been described at times as different varieties or species. All on Atlantic coasts of Europe are now considered to be a single species by most authorities, such as Warén (1996), Cadée (1998), Wigham (2017) and the World Register of Marine Species (accessed November 2017).
Two types were distinguished by Rehfeldt (1968) and Verduin (1982) on the sole basis of the dimensions of the protoconch and the associated larval development (see reproduction in Habits and Ecology section below). Warén (1996) found examples that combined elements of the two reproductive methods and wrote:
‘the development of R. membranacea is not so much an ‘either/or’ situation – – – but perhaps more of a continuum of adaptations to local conditions. – – – it is impossible to give any general distinctive characters [of the two types] which hold for all localities (except the protoconch). – – – there are problems with Mediterranean specimens, although the species seems to continue into the Mediterranean.’
Cadée (1998), Wigham (2017) and WoRMS concur that it lives in the Mediterranean. As some recorders in the Mediterranean regard specimens there to be a separate species, their details have been put separately in the ‘Key Identification Features’ section, images 34 Rissoa membranacea to 41 Rissoa membranacea with notes on their similarities with, and differences from, west European specimens. Comparative DNA sequencing is desirable to satisfy all concerned.
Shell description
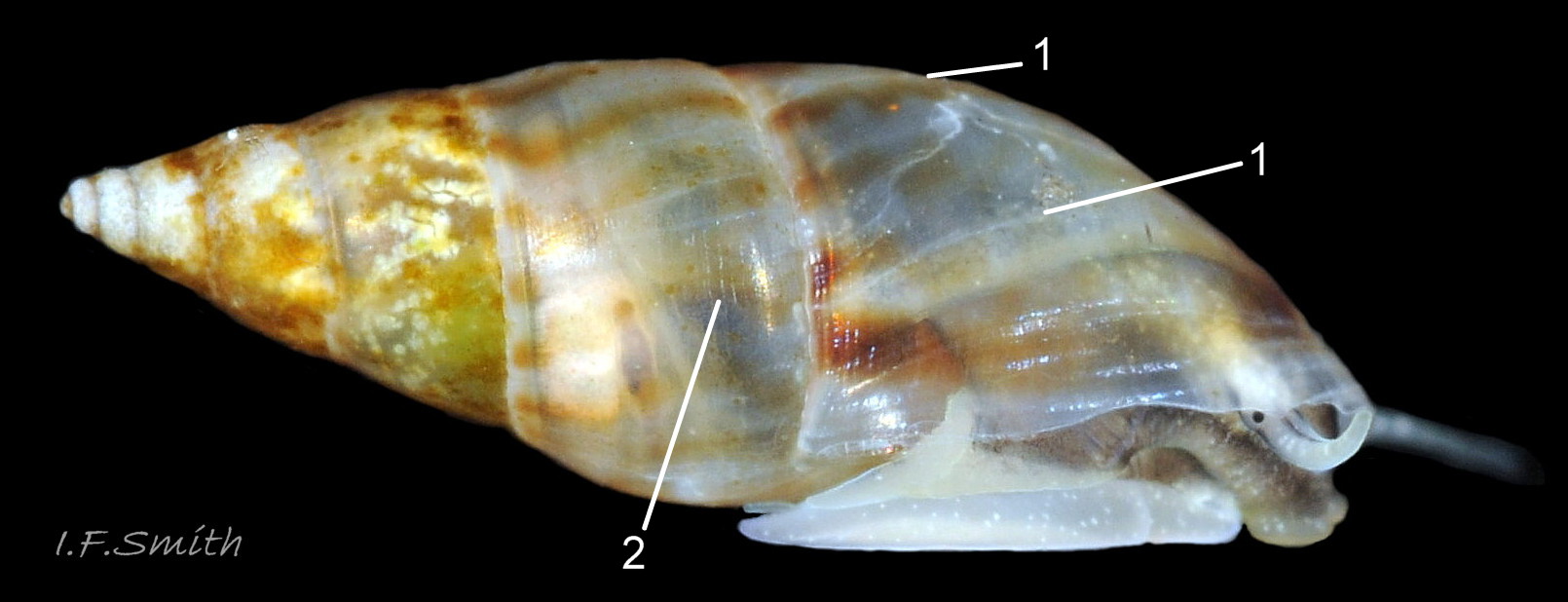
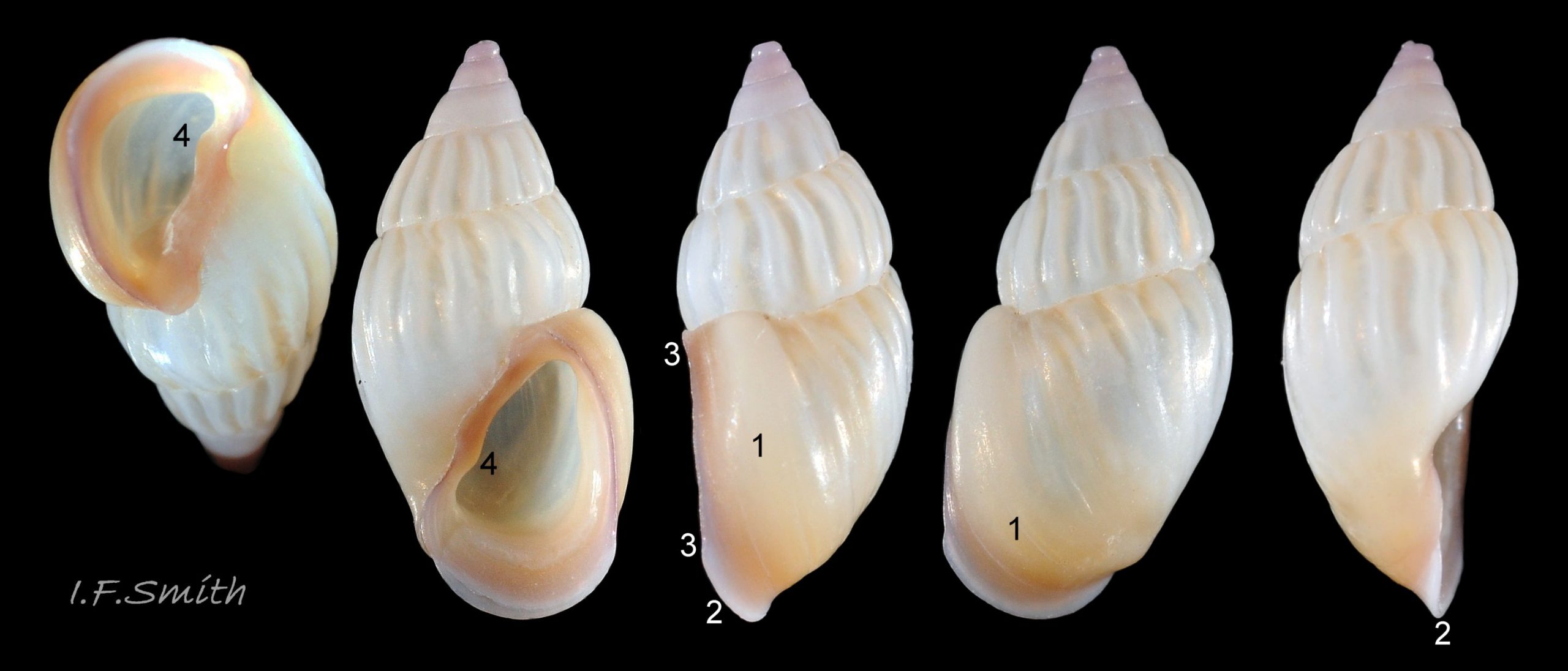
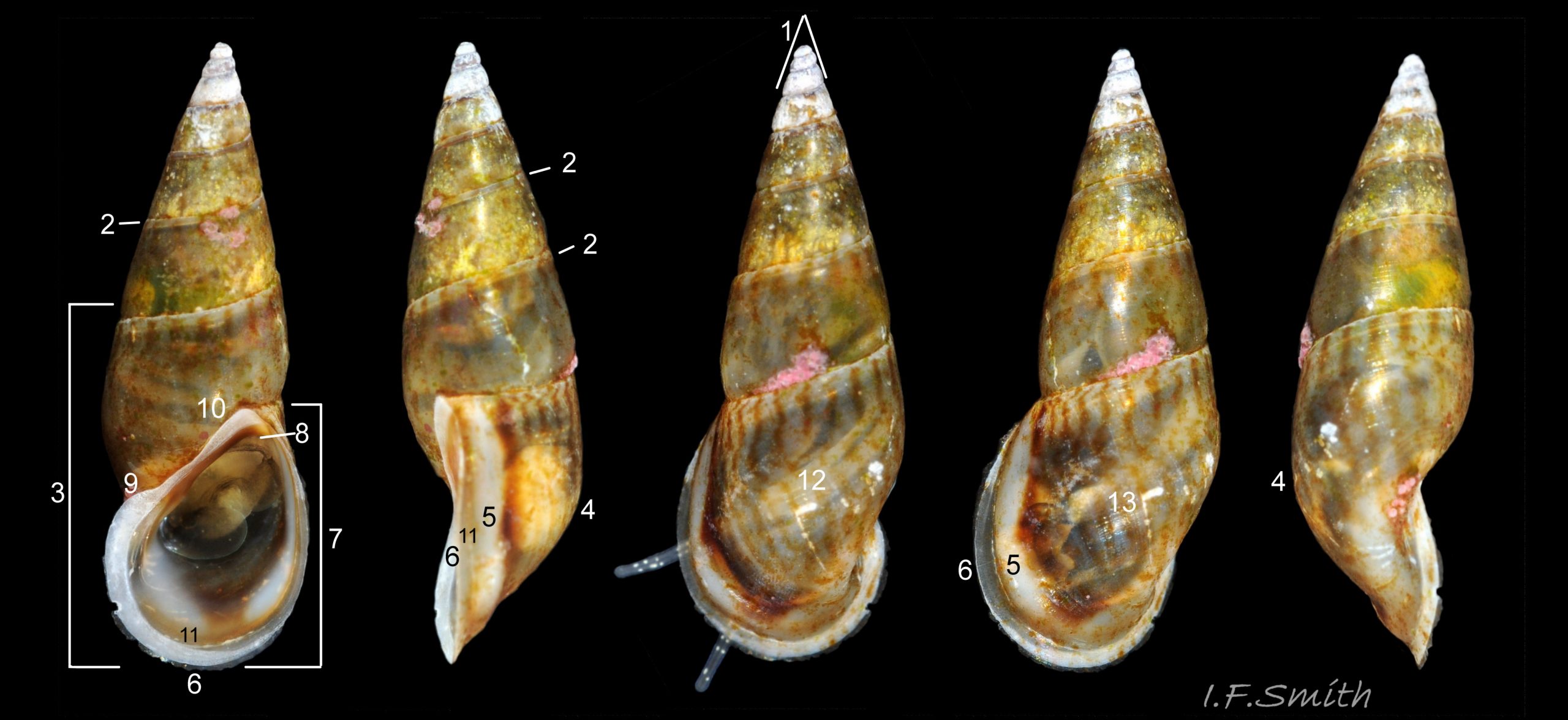
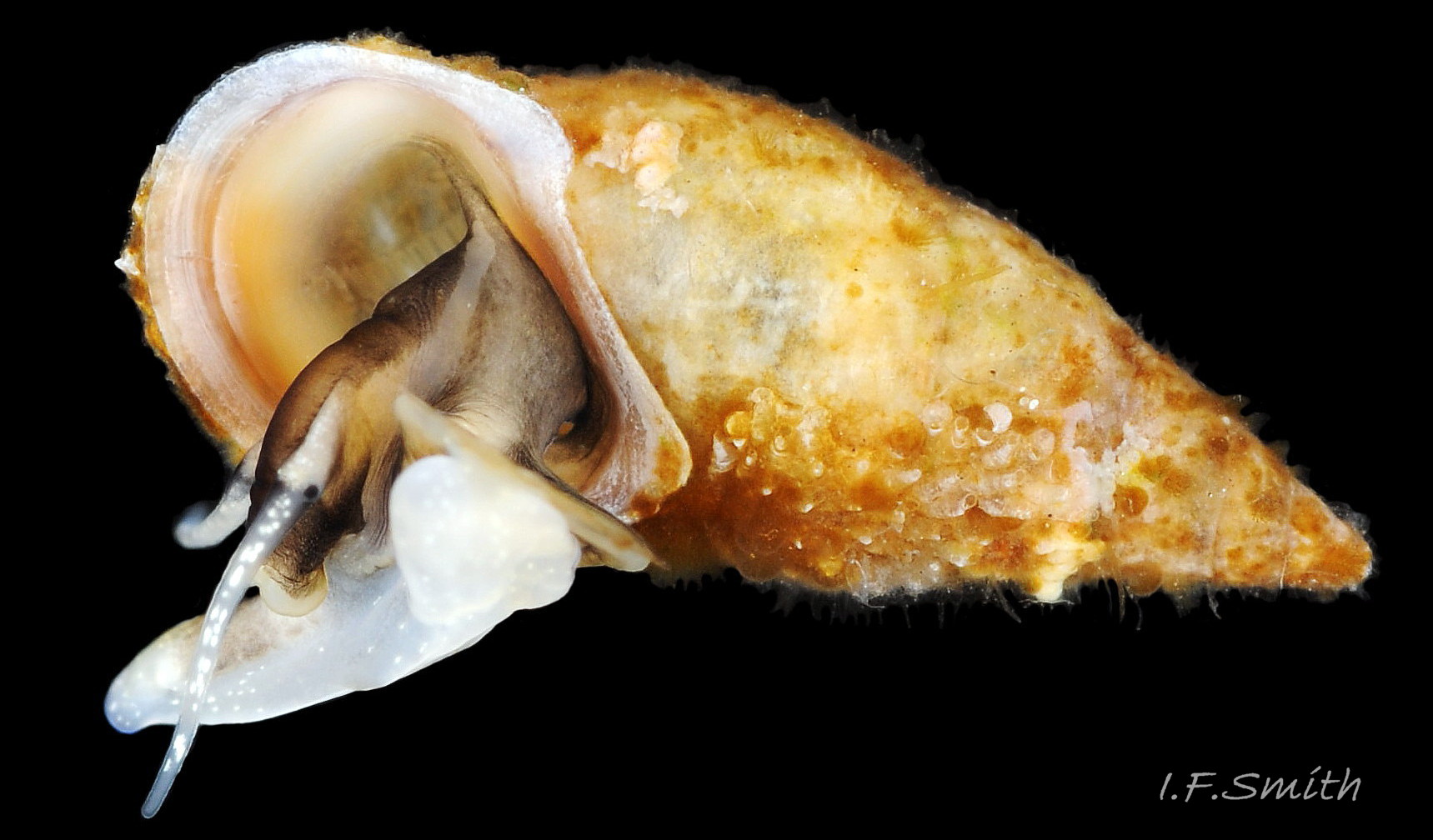
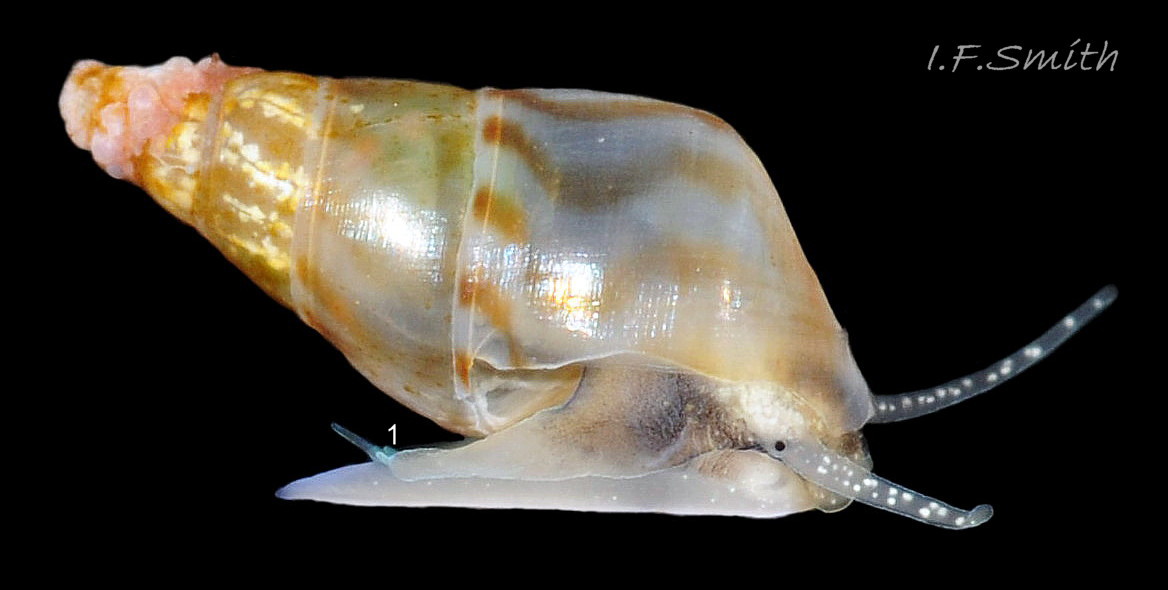
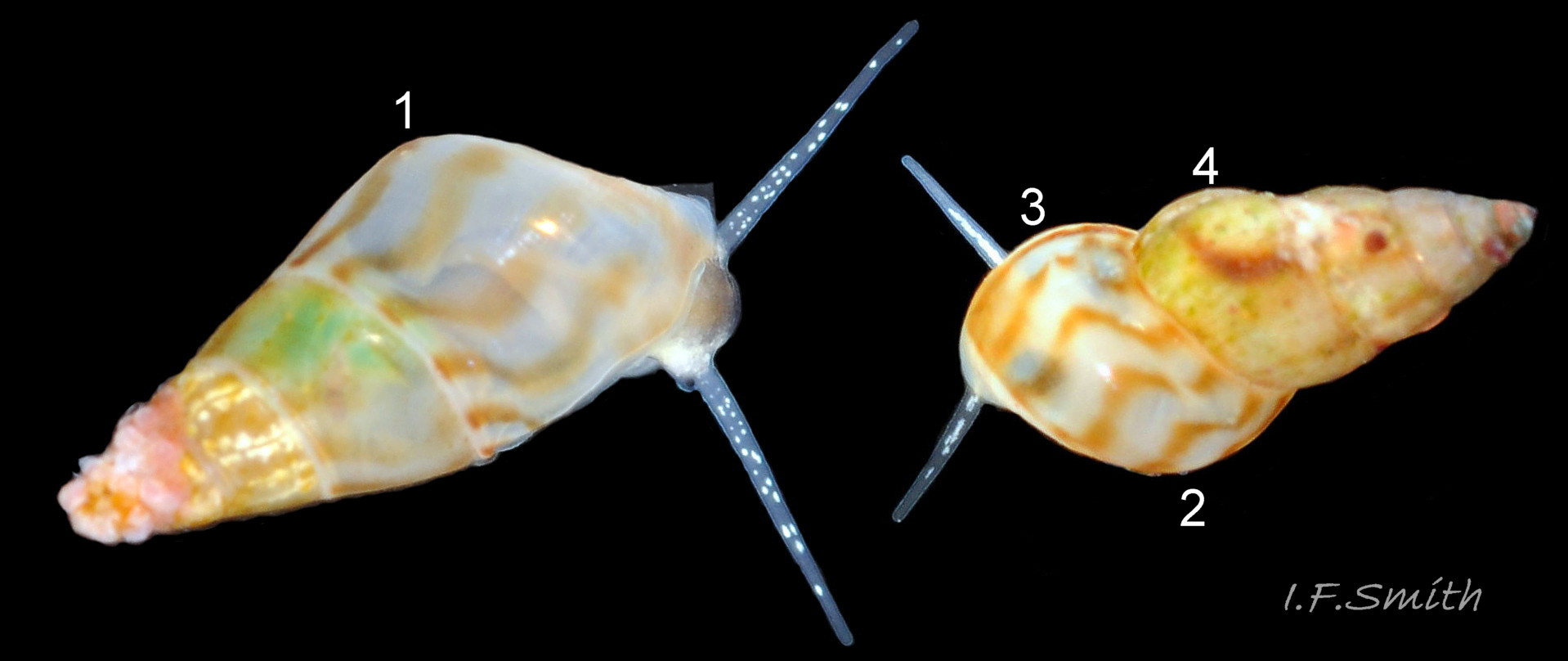
Up to 9mm high and 3mm broad; exceptional shells in Lofoten Is., Norway to 13.4mm high (Verduin, 1982). Shell walls thin and translucent 01 Rissoa membranacea to solid and well calcified 02 Rissoa membranacea. Profile tall with 8 to 9 adult whorls 03 Rissoa membranacea or fairly tall with 5 to 7 whorls 02 Rissoa membranacea; body whorl 40% to 65% 03 Rissoa membranacea of shell height , with apical angle about 34º 03 Rissoa membranacea to 45º 02 Rissoa membranacea. Whorls fairly flat 04 Rissoa membranacea to tumid. Suture is emphasised by a subsutural collar of altered pattern and gradient 03 Rissoa membranacea. Periphery of body whorl is smoothly rounded on adults 03 Rissoa membranacea & 04 Rissoa membranacea but angulated on young 05 Rissoa membranacea. Sculpture: smooth surface has fine growth lines and barely-visible, microscopic, spiral striae 01 Rissoa membranacea . Others have wave-like costae (ribs) and may have a slight tooth-like protuberance on the columella 02 Rissoa membranacea. Fully grown adults usually develop a white, labial varix set back from the palatal (outer) lip 03 Rissoa membranacea. The ground colour is white 04 Rissoa membranacea; but, when live, the shell is often tinted horn-colour or greenish by the periostracum and the body seen through the translucent shell 04 Rissoa membranacea Sometimes, there are sinuous, orange-brown, costal bands on most whorls except those near the apex 04 Rissoa membranaceaand there may be purple or lilac around the aperture 02 Rissoa membranacea & 10 Rissoa membranacea. The aperture varies oval 04 Rissoa membranacea to D-shape 03 Rissoa membranacea; about 35% to 50% of shell-height ; adapical angle about 70º. The lips form a continuous peristome which is unobtrusive on the parietal area of juveniles 05 Rissoa membranacea but well developed on mature adults 03 Rissoa membranacea. The labial varix on mature specimens is set back from the thin, curved, outer (palatal) lip, so does not thicken it. On fully mature adults, the entire lip everts to form a flared aperture 03 Rissoa membranacea. It reflects onto the body whorl in the columellar and parietal areas and may form a very slight umbilical groove. Internally, the aperture is whitish, translucent 04 Rissoa membranacea, sometimes becoming more opaque at maturity, often with a brown band between the varix and lip both externally and internally 03 Rissoa membranacea. The flimsy operculum is pale horn-colour, semi-transparent 06 Rissoa membranacea, showing the pale underlying opercular disc 07 Rissoa membranacea . It forms an excentric spiral with the nucleus near the columellar lip when withdrawn.
Body description
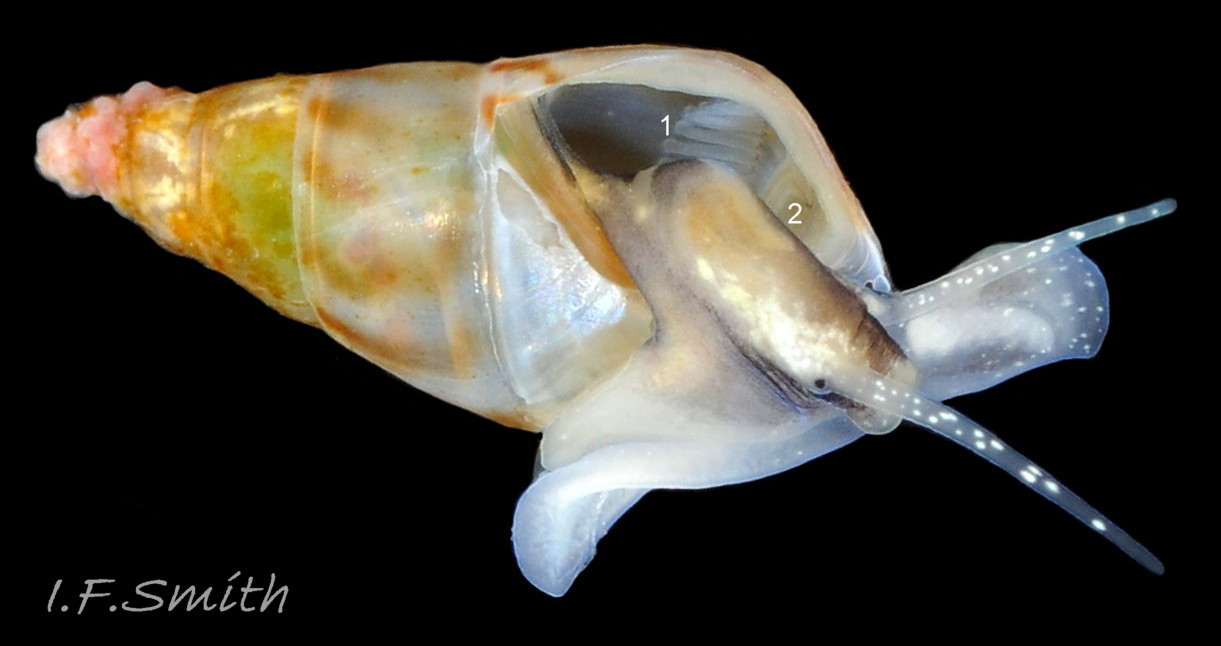
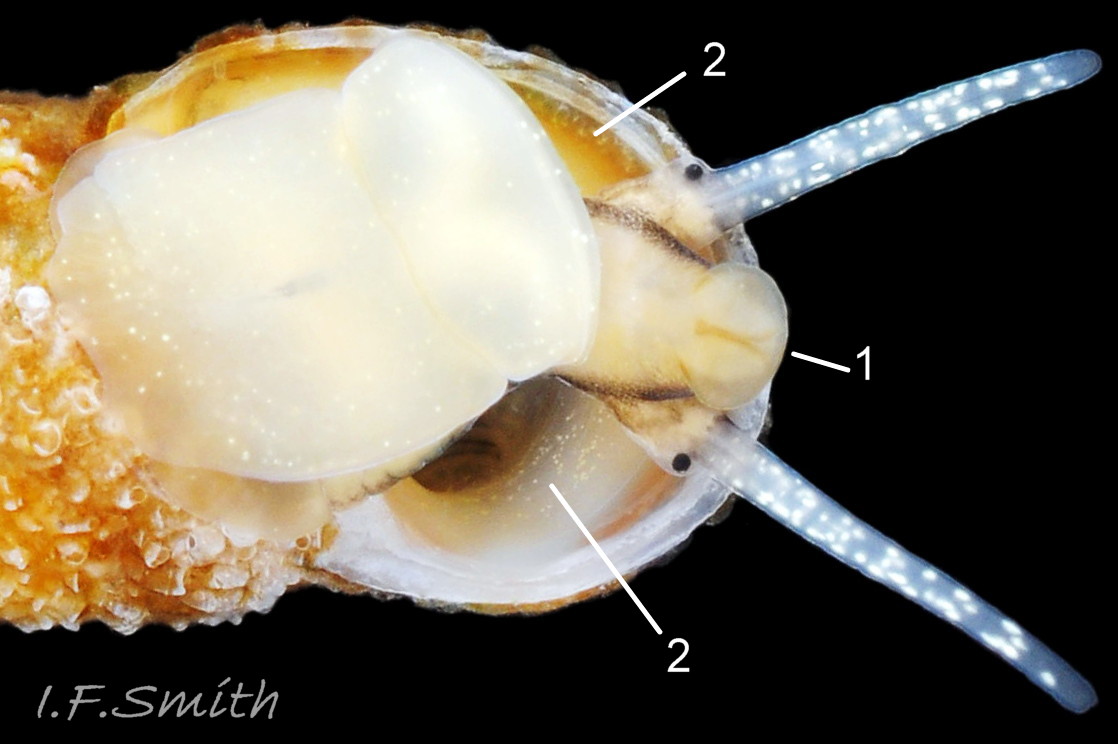
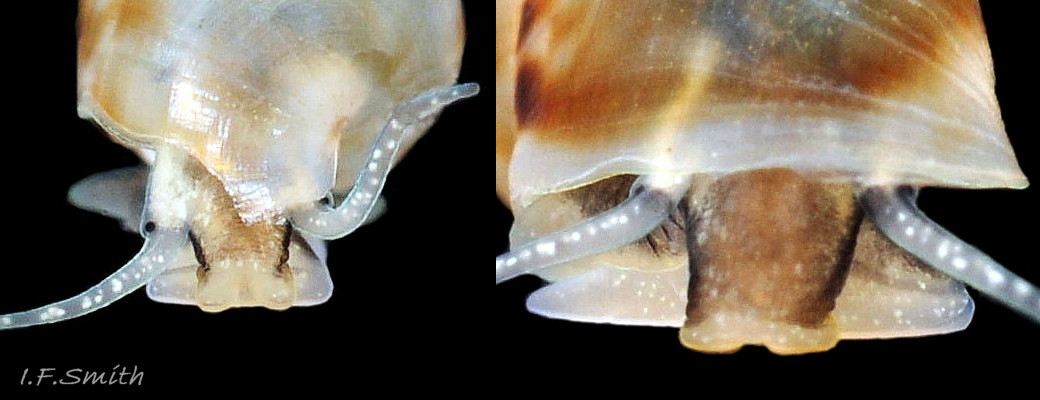
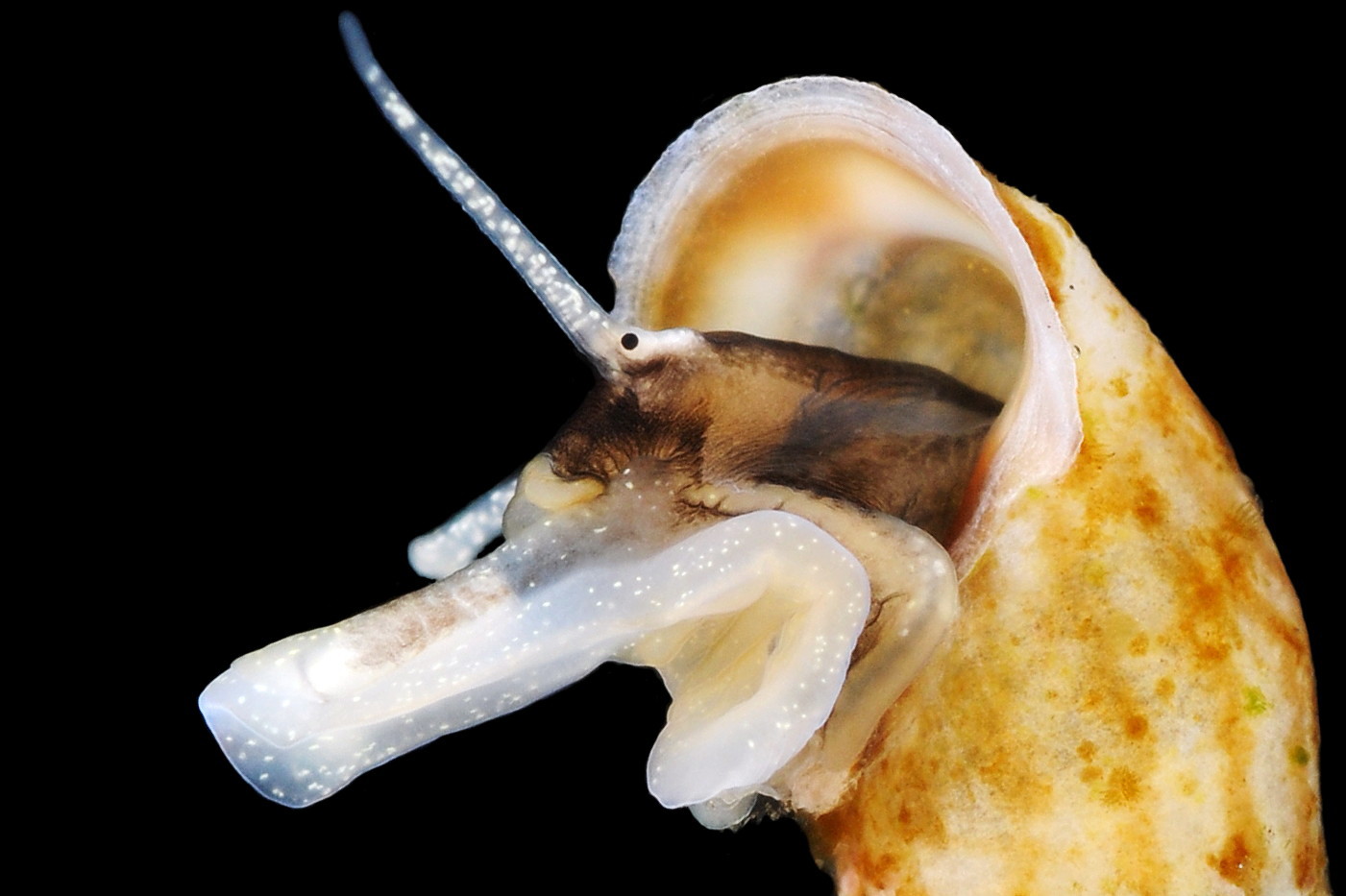
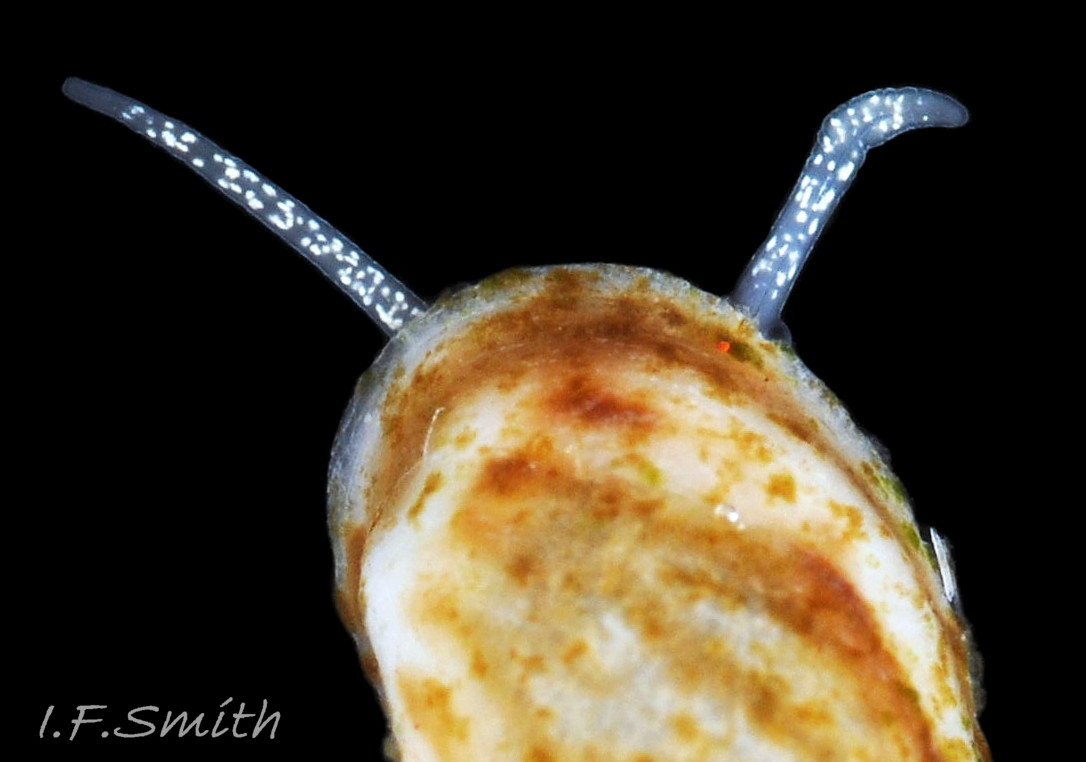
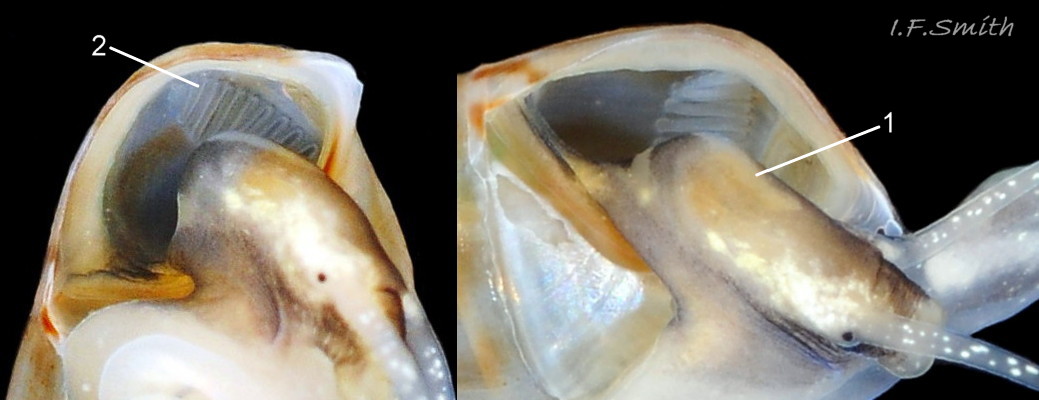
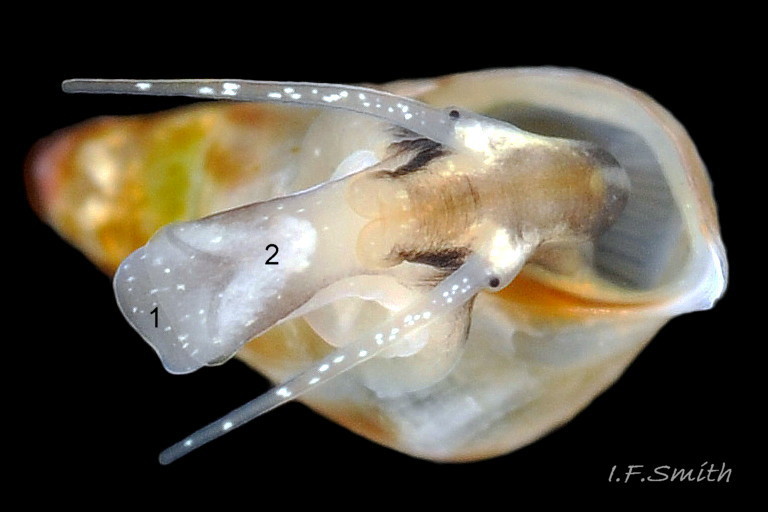
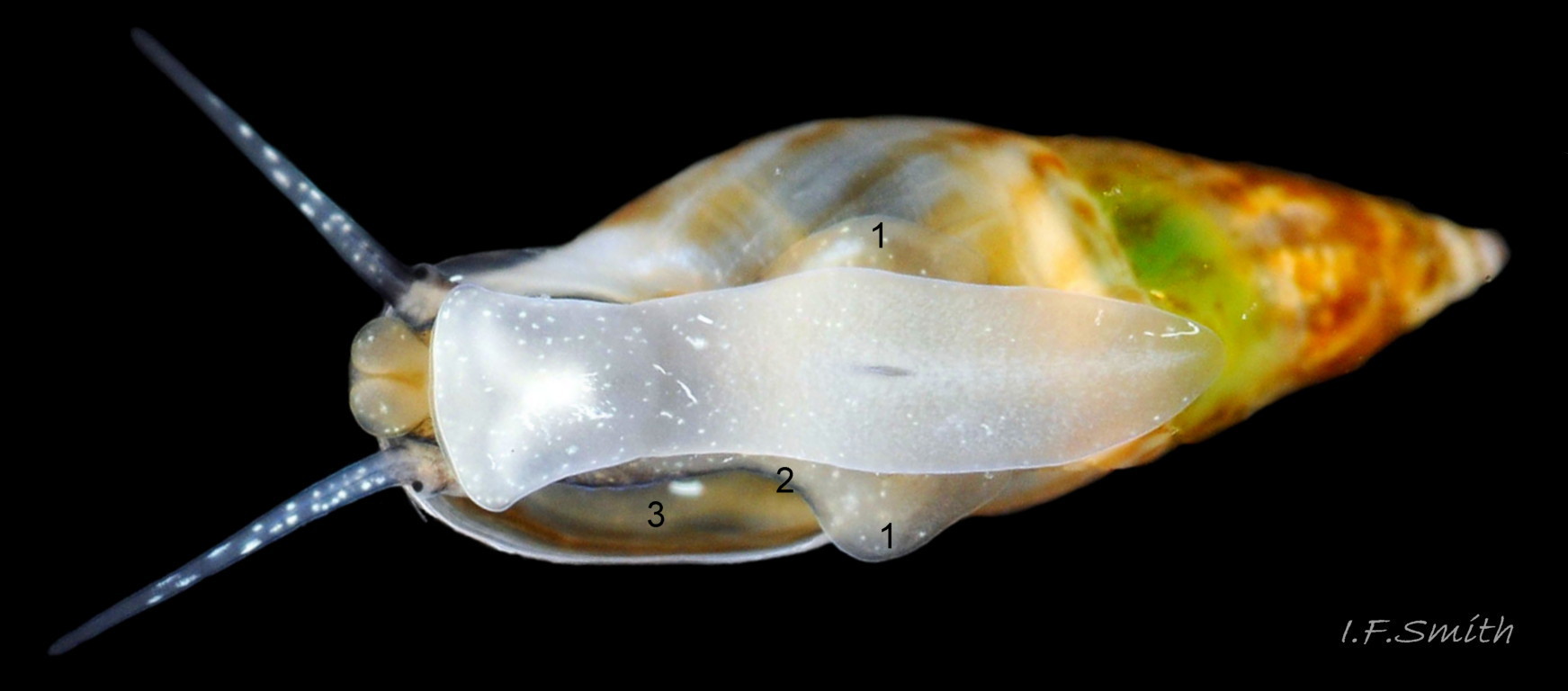
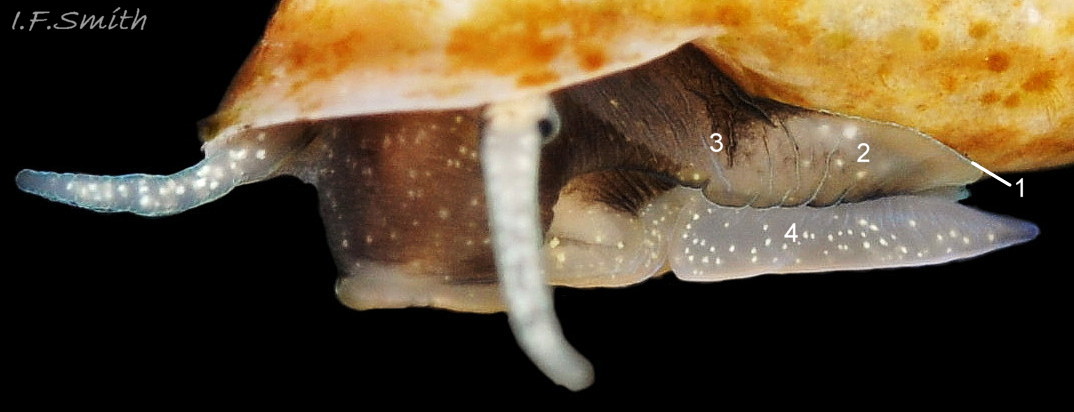
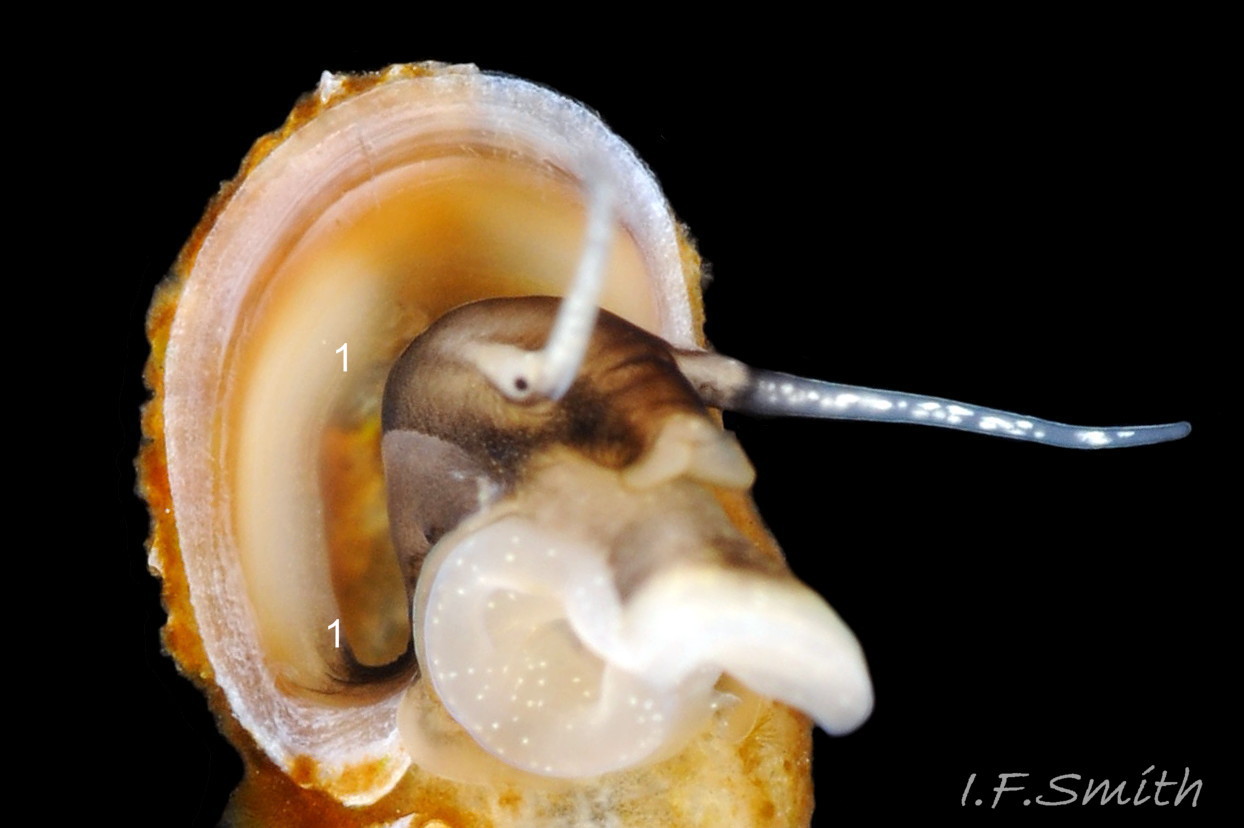
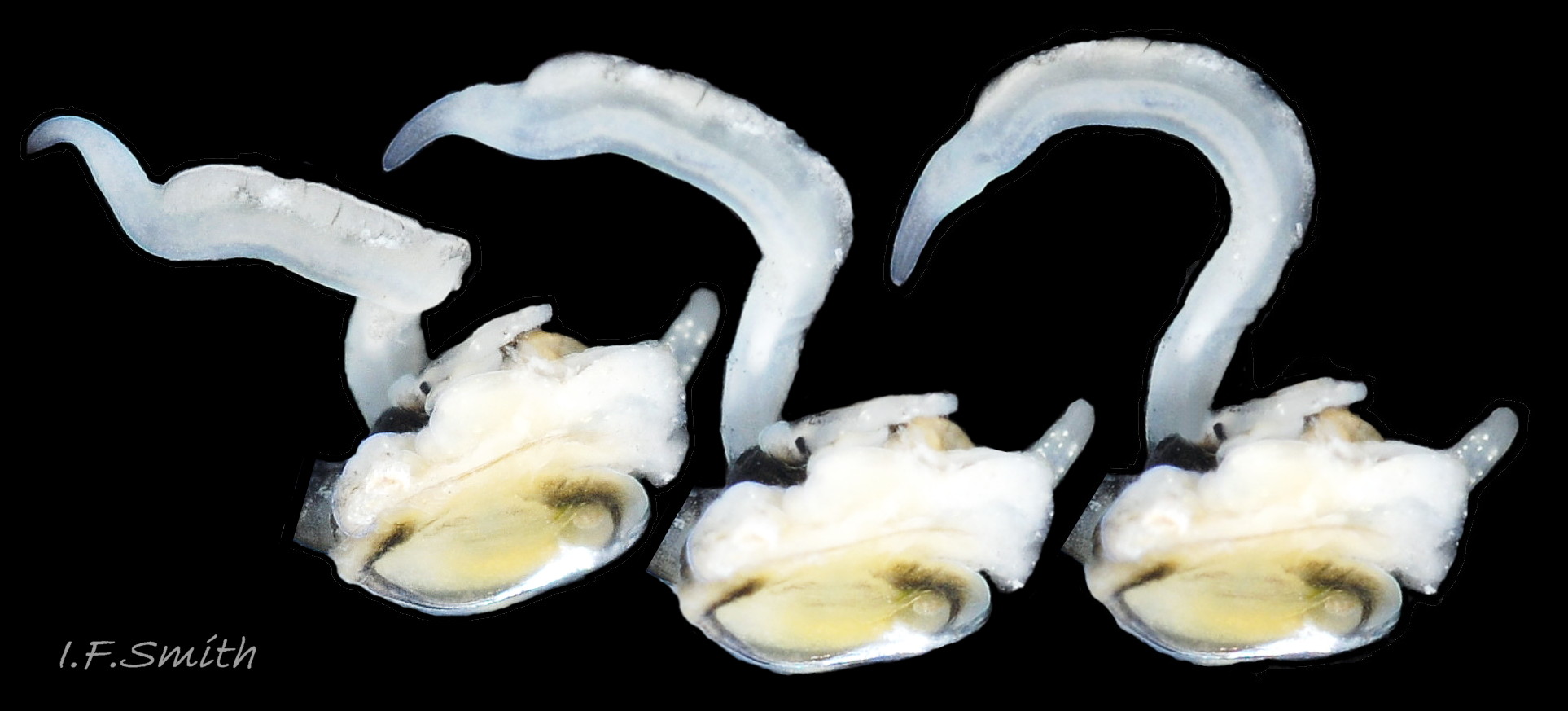
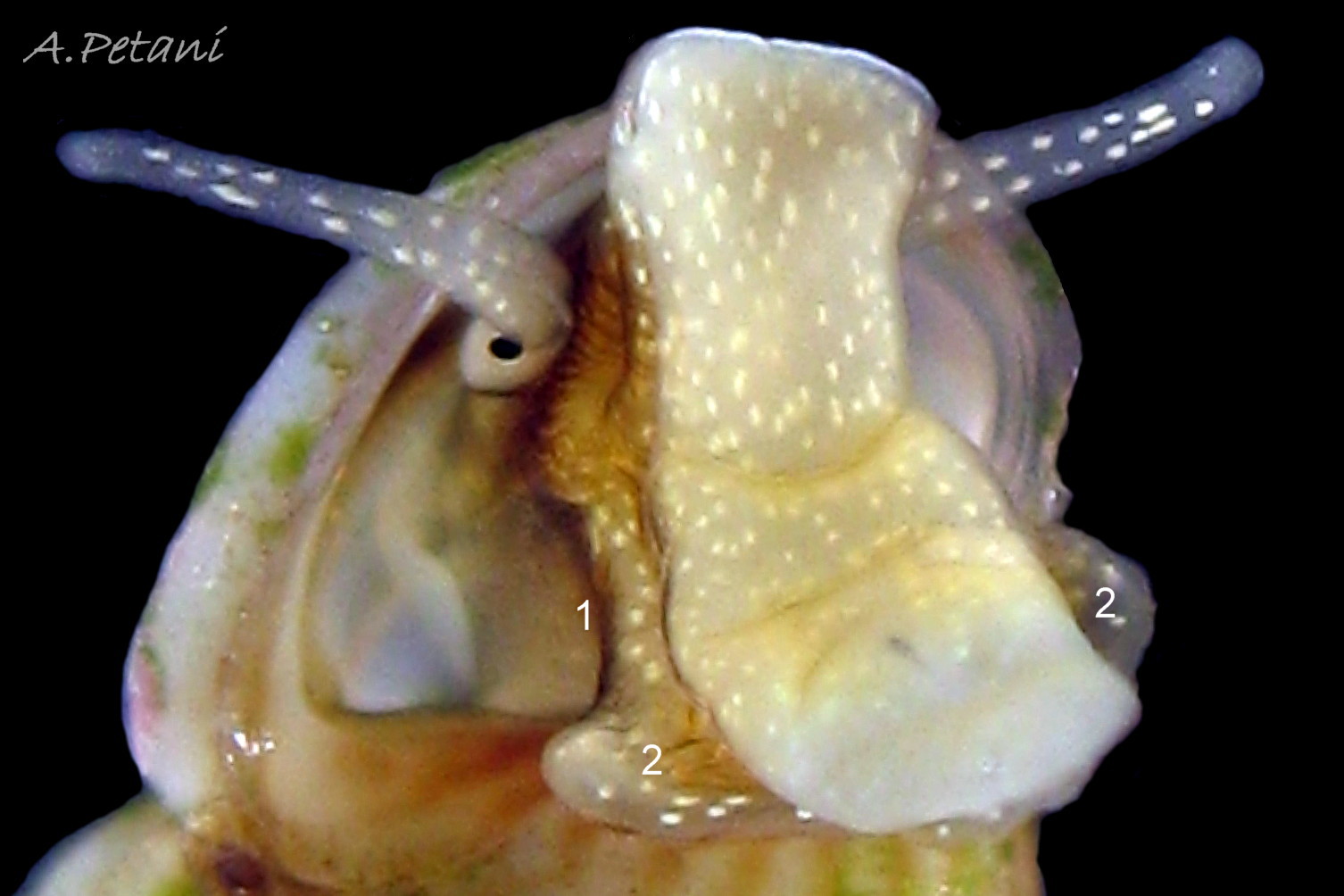
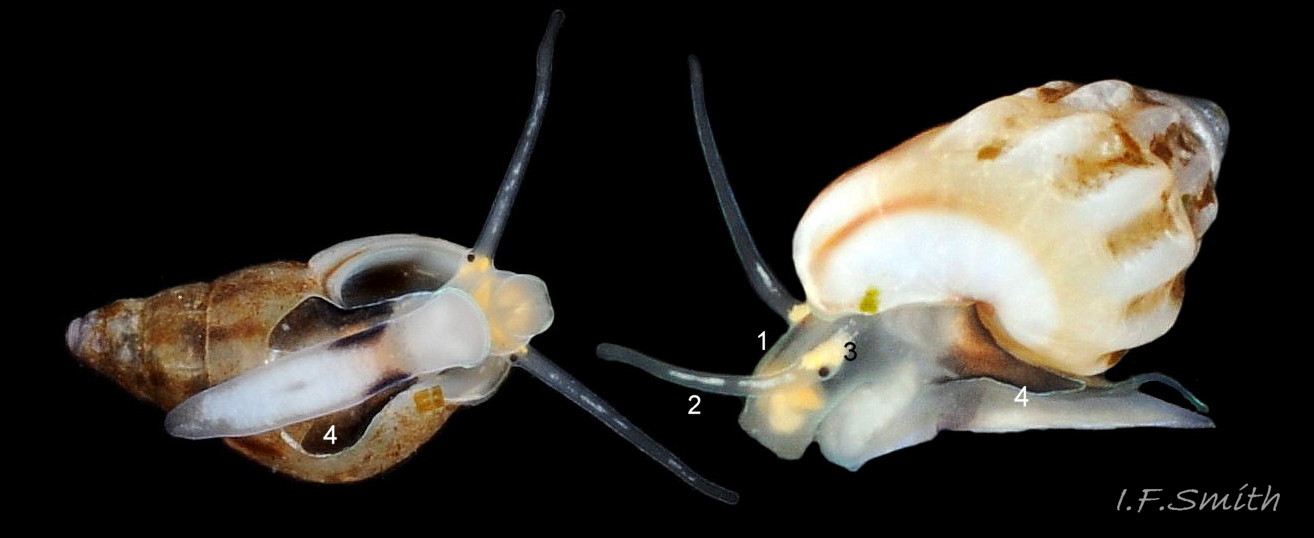
The ground colour of the flesh is whitish, translucent, and it is extensively covered by various shades of grey to brown-black pigment dorsally, with opaque white spots 08 Rissoa membranacea. The intensity of the pigment varies, but its pattern of distribution on pale specimens is similar to that on darker ones 10 Rissoa membranacea& (Biopix image) www.biopix.com/photos/Rissoa-membranacea-00002.JPG . The snout is bifid 11 Rissoa membranacea with a longitudinal slit mouth in the terminal cleft 12 Rissoa membranacea. It is pigmented darkest laterally 13 Rissoa membranacea & 14 Rissoa membranacea, but the difference from the dorsum is lost on the darkest-overall specimens 15 Rissoa membranacea. The snout has an unpigmented, yellowish-white ventral face 12 Rissoa membranaceaand tip 11 Rissoa membranacea. The spots are less crowded on younger specimens, but are usually not arranged in one or two well defined lines 17 Rissoa membranacea. A bulge at the base of each tentacle 11 Rissoa membranacea & 15 Rissoa membranacea bears a black eye with a variably sized, opaque, pure-white, dorsal patch behind it. Behind the tentacles the dorsal colour of the snout continues on the body dorsally 18 Rissoa membranaceawith a whitish, medial, longitudinal band in the posterior half 19 Rissoa membranacea. The sides of the body are pigmented, but less intensely than the dorsum, and there is a translucent, whitish, slender triangle dorso-laterally that tapers towards the posterior 08 Rissoa membranacea. All examined specimens from Orkney had pigment that varied from grey 20 Rissoa membranacea to brown-black 18 Rissoa membranacea; older ones were usually darker than juveniles. Pale snouts are still usually darker laterally than dorsally (Fretter & Graham, 1978) 10 Rissoa membranacea The foot is translucent white with some opaque white spots and, dorsally, pale grey and/or pale-brown shading 15 Rissoa membranacea. The anterior is deeply bilaminate, lacking dorsal pigment in front of the opaque, white, ‘V’ shaped anterior pedal mucous gland 20 Rissoa membranacea. On darker specimens, the surface pigment may partially obscure the gland 08 Rissoa membranacea. The anterior quarter of the sole is axehead shaped, and the posterior three quarters are spearhead shaped 21 Rissoa membranacea. It is translucent white with opaque white spots, and the internal opaque white, ‘V’ shaped anterior pedal mucous gland is visible; it opens to the anterior within the bilaminate anterior. The opening of the posterior pedal mucous gland is just behind the centre point, and has a medial groove running from it to the posterior tip. The columellar muscle, pigmented externally as the body, connects the columella of the shell to the operculum. The end of the whitish interior of the muscle is visible as the opercular disc through the horn coloured operculum 07 Rissoa membranacea. The disc extends beyond the operculum as prominent, translucent, white opercular lobes 22 Rissoa membranacea, unpigmented except proximally where the thick disc merges with the columellar muscle 23 Rissoa membranacea & 24 Rissoa membranacea. A long, translucent-white, metapodial tentacle extends obliquely upwards from below the posterior of the opercular disc 25 Rissoa membranacea; sometimes a smaller tentacle is visible on each side of it 26 Rissoa membranacea. The translucent mantle is variably coloured, often with opaque white spots that may be difficult to see on whitish areas 12 Rissoa membranacea The mantle edge is often brownish, and the posterior of the mantle cavity roof is greyish 08 Rissoa membranacea . A translucent white pallial tentacle sometimes protrudes from the adapical angle of the shell-aperture 28.2 Rissoa membranacea. Within the mantle cavity there are a large penis (on males) 27 Rissoa membranacea, 28 Rissoa membranacea & 22 Rissoa membranacea, a short, grey-white ctenidium with relatively few short stout filaments 19 Rissoa membranacea & 28.2 Rissoa membranacea and an osphradium 09 Rissoa membranacea. The penis is recurved to fit the shorter mantle cavity 28. 28.1 Rissoa membranacea. It is translucent white with a dark genital duct running through it (when alive) and is attached a short distance behind the right tentacle 28. 28.2 Rissoa membranacea. When out of the mantle cavity, it can assume a variety of shapes 28.3 Rissoa membranacea. The proximal 75% is almost uniform in width and appears to have white glands along the dorsal edge. The distal 25% is a tapering, glandless filament that narrows to the point where sperm is emitted 28.3 Rissoa membranacea. The dark areas on the penis vary in extent on the same individual.
Habits and ecology
Before 1934, R. membranacea was widespread and common in NW Europe, primarily on the flat strap-like leaves of the marine vascular plant Zostera marina 29 Rissoa membranacea. From 1934 and through the 1940s, disease caused the destruction of most European Zostera beds and a consequent drastic reduction, or extinction, of R. membranacea populations at most sites, though the snail persisted in small numbers at places such as Isefjord, Denmark on filamentous algae, fucoids, and even on mud at 7 to 12m depth. Many Zostera beds have now recovered, but R. membranacea has failed to re-establish itself at some, perhaps because it became locally extinct, and its poor dispersal ability has prevented recolonization from elsewhere 30 Rissoa membranacea. R. membranacea became extinct in the Dutch, German and Danish Wadden Sea with the loss of Zostera. In 1997, only dead shells could be found (Cadée, 1998), and the snail is still extinct there as increased turbidity impeding photosynthesis, changed water depths and movements, and salinity fluctuation have prevented the return of Zostera beds (Heide, Katwijk & Geerling, 2006).
Currently (2017) in Northern Europe it lives on some Zostera beds in shallow water sites sheltered from wave action, with non-turbid water, and salinities ranging from fully marine down to 12 p.p.t. (or 7p.p.t for short periods). It lives from LWST to about 15 m depth. It feeds by grazing detritus, diatoms and other microphytes from the surface of Zostera 31 Rissoa membranacea & 32 Rissoa membranacea and plants with similar structure, such as Posidonia in the Mediterranean (image M. Mattana flic.kr/p/nPiS2k ), and sometimes other seaweeds. Grazing probably benefits the plants by keeping them clean for photosynthesis but, when the population is dense, they do feed on the substance of the plants. Ovoid faecal pellets are produced 32.1 Rissoa membranacea. Populations are drastically reduced in winter in northern Europe when Zostera leaves die and drop; only a small portion survives to reach maturity and breed in the following summer (van Goor, 1919, in Cadée 1998).
Shell sculpture apparently responds to small changes in environment in a similar way to that described by Wigham (1975a) for Rissoa parva (Fretter & Graham, 1978) with ribbed forms tending to occur more frequently sublittorally 33 Rissoa membranacea and in warmer environments 32 Rissoa membranacea
The respiratory inhalent current comes into the mantle cavity at the left of the head and has the water quality tested by the osphradium before reaching the grey-white ctenidium with substantial filaments 19 Rissoa membranacea. The exhalent current passes from the mantle cavity at the right of head. Locomotion: The anterior pedal mucous gland discharges within the bilaminate anterior edge of the sole 20 Rissoa membranacea and spreads mucus to lubricate creeping. The posterior pedal gland secretes adhesive mucus that is shaped by a medial groove on the posterior half of the sole 21 Rissoa membranacea into strong threads that harden on contact with sea water and are used to help secure the snail in its movement around plants.
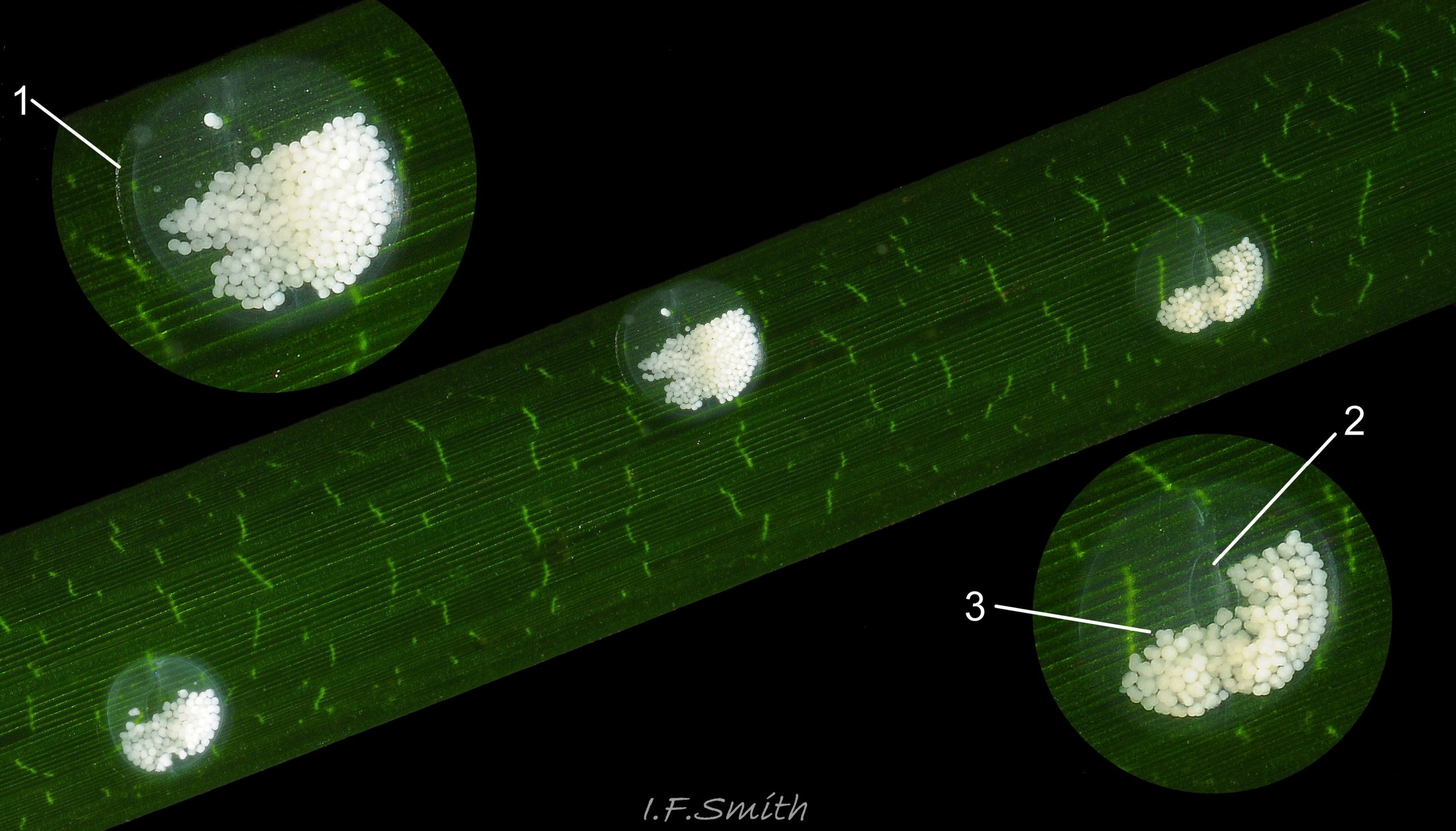
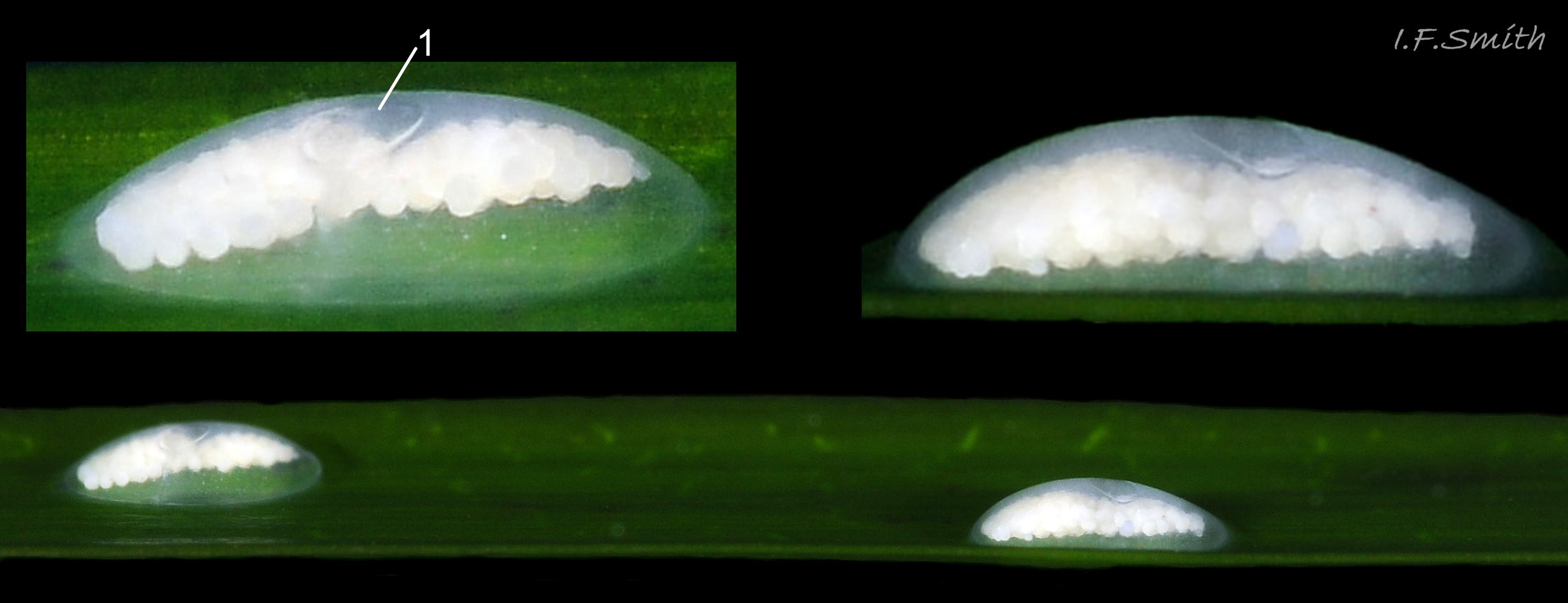
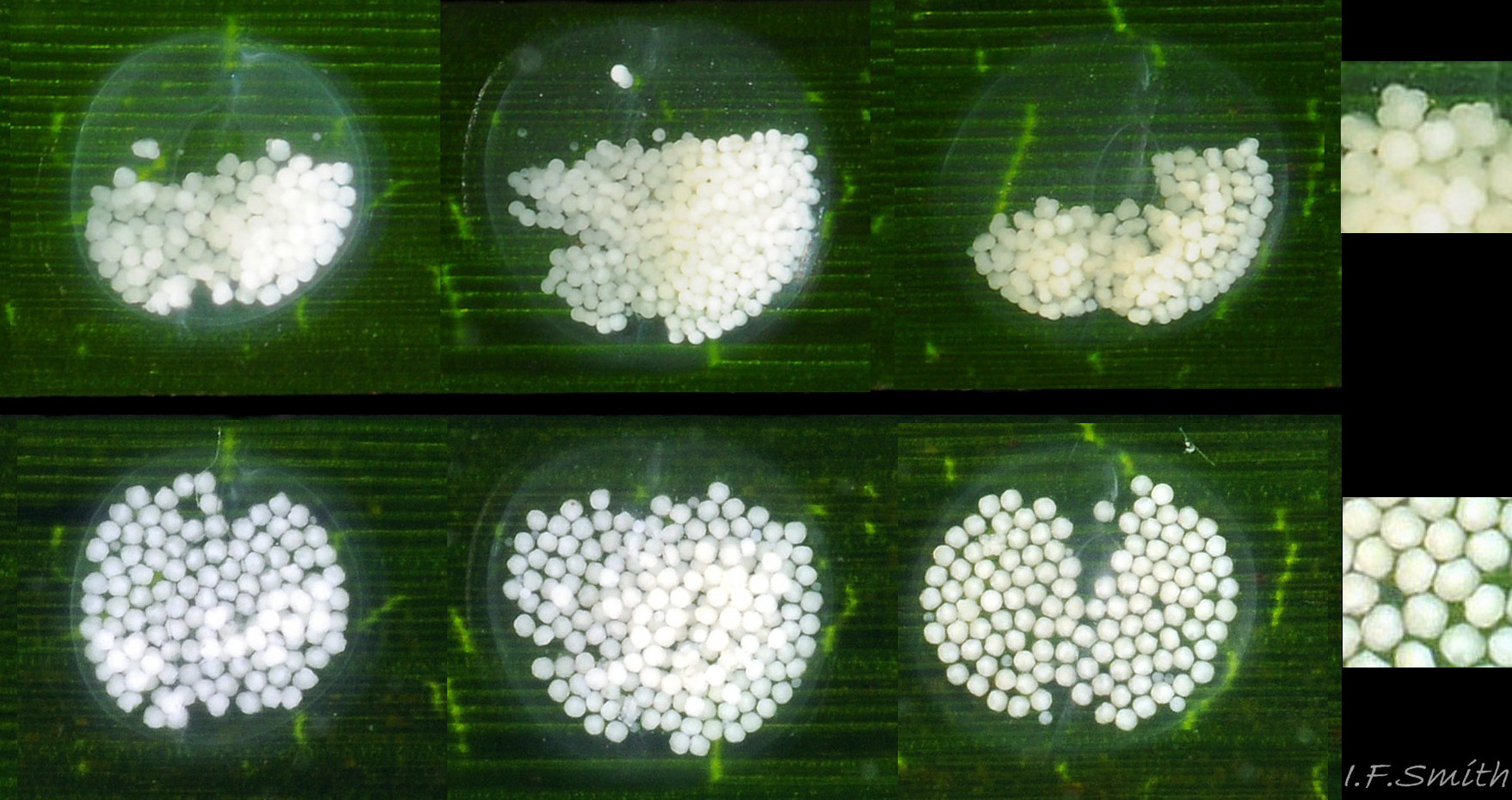
Reproduction: Development of a labial varix indicates sexual maturity. The male’s recurved penis 28. 28.1 Rissoa membranacea performs internal fertilization. Egg capsules, about 1.5mm in diameter, are laid usually on a leaf of Zostera when the temperature is over 8ºC 30. 30.1 Rissoa membranacea. The capsule is a low dome with a flat rim adhering to the leaf and a central lanceolate opening covered by a membrane that ruptures when the eggs hatch so the young can escape 30. 30.2 Rissoa membranacea.The capsule is colourless transparent, almost invisible so often all that is noticed is the 40 to 400 opaque, white ova within 31 Rissoa membranacea & 30.3 Rissoa membranacea. Females die soon after spawning and the eggs hatch in about 5 weeks (Warén, 1996). Post-embryonic larvae are present in Northern Europe during the summer; in Denmark, from May to September when the water is above 10ºC, (Rasmussen, 1973). Breeding is all year on the south coast of England, France and places further south (Fretter & Graham, 1978), and there are reports of egg capsules in most months even from northern localities (Warén, 1996). Differences in development have been distinguished between two types, named variously by different authors:
1) Type: A (Rehfeldt, 1968); planktotrophic (Cadée, 1998); Rissoa labiosa (Verduin, 1982);
Distribution: Norway to the Mediterranean and Black Sea (Verduin, 1982).
Egg capsules: 30 to 400 white eggs per capsule, egg diameter 0.09 to 0.1mm (Rasmussen, 1973).
Larvae: planktotrophic veligers for a short period, free swimming, feeding on plankton among Zostera. Settle from plankton onto Zostera when shell height 0.35 to 0.45mm (Warén, 1996)
Protoconch on adult: diameter less than 0.25mm, narrowly twisted, distinctly smaller than on type B. No consistent difference, other than protoconch, from shell forms of type B.
2) Type: B (Rehfeldt, 1968); lecithotrophic (Cadée, 1998); Rissoa membranacea (Verduin, 1982);
Distribution: restricted to United Kingdom, St Malo in Bretagne, sheltered fjords, straits and bays in Zealand and eastern Jutland in Denmark (Warén, 1996) and, former population now extinct, the Dutch-German-Danish Wadden Sea (Cadée, 1998).
Egg capsules: domed, thick-walled capsules. 18 to 75 yellowish eggs per capsule, egg diameter 0.12 to 0.125mm (Rasmussen, 1973).
Larvae: lecithotrophic, all larval development confined within capsule feeding on yolk of own egg and, extending into adelphophagy, the yolks of non-developing ‘nurse’ eggs. Emerge from capsule as crawling small versions of adult with shell diameter 0.28 to 0.36mm (Rehfeldt, 1968).
Protoconch on adult: diameter more than 0.25mm, distinctly larger than on type A. No consistent difference, other than protoconch, from shell forms of type A.
Growth is rapid after metamorphosis. Shells were observed in the former Zuiderzee by van Goor (1909, in Cadée, 1998) to grow from 1mm high in July to near maximum size for the locality of 5.5 to 9mm by September. Smidt (1938, in Cadée, 1998) found a similar rapid growth from 1mm in early August to an early September height of 3.5 to 4mm, the local maximum, in the Øresund, Denmark. Maximum numbers occur in late summer in northern Europe; 100 000/m² were recorded in Limfjord, Denmark in 1918 (Petersen, 1918, in Fretter & Graham, 1962). In northern localities, only a small part of the population survive the winter loss of leaves by Zostera to breed the following summer. The lifespan is a little over a year as adults die soon after spawning.
Apart from the protoconch, the diverse shell-forms do not correspond with development types A or B. Shell form is probably influenced by genetic isolation of populations and a cocktail of environmental influences including temperature, salinity and wave exposure.
Taxonomy
Warén (1996), Cadée (1998), Wigham (2017) and the World Register of Marine Species (accessed November 2017) regard Rissoa membranacea in western Europe as a single species with two different types of larval development which may be adaptations to local environmental conditions. Rehfeldt (1968) and Verduin (1982) considered the differences in protoconch sufficient to regard the two types as separate species. The situation in the Mediterranean is uncertain. Some regard the population there as different from the Atlantic R. membranacea of two development-forms and refer to it as Rissoa labiosa (Montagu, 1803) which is regarded as a synonym of R. membranacea by WoRMS. Another similar species, Rissoa paradoxa (Monterosato, 1884) lives on the North African coast.
Key identification features
Rissoa membranacea from north-east Atlantic.
1: maximum height usually 9mm in Britain, 13.4mm recorded in northern Norway.
2: shell varies thin and unribbed 04 Rissoa membranacea. to thick and ribbed 02 Rissoa membranacea. . Ribs sometimes overhang the suture 33 Rissoa membranacea. Sometimes a slight tooth-like protuberance on the columella. Protoconch large or small.
3: tall, or moderately tall, spire with subsutural collar 03 Rissoa membranacea.
4: entire lip everts to form a flared aperture on mature adults 03 Rissoa membranacea.
5: flesh of head and body whitish, translucent, extensively covered by various shades of grey to brown-black pigment dorsally with opaque white spots 08 Rissoa membranacea. & 09 Rissoa membranacea.
6: cephalic tentacles translucent white with crowded, randomly arranged, opaque, white spots 16 Rissoa membranacea.
7: variably sized, opaque, pure-white, dorsal patch behind each eye 11 Rissoa membranacea. & 15 Rissoa membranacea.
8: foot is translucent white with some opaque white spots and, dorsally, pale grey and/or pale-brown shading 15 Rissoa membranacea.
9: opercular lobe pale apart from some dark pigment proximally 22 Rissoa membranacea. & 23 Rissoa membranacea.
10: ELWS, mainly on Zostera 29 Rissoa membranacea album. in N.W. Europe.
Rissoa membranacea Mediterranean forms. (From Croatia leg. J. Prkić and A. Petani, and from Italy leg. F. Vitale.)
Some workers record Mediterranean forms as a separate species, R. labiosa (Montagu, 1803), which is regarded as a synonym of R. membrancea by WoRMS.
= points of apparent difference from Atlantic specimens and images examined from Orkney, Galway and Bretagne. In such a varied species, it is possible that specimens might be found at other Atlantic sites with the features.
1: height usually 4 to 6 mm, maximum c. 8 mm 34 Rissoa membranacea.
2: shells strong, thick and ribbed 35 Rissoa membranacea. no smooth ones found by J.P. and A.P. in Croatia. Ribs sometimes overhang the suture 37 Rissoa membranacea, Often, a slight tooth-like protuberance on the columella. Protoconch is always small (Verduin, 1982).
3: tall, or moderately tall, spire with subsutural collar 36 Rissoa membranacea,
4: entire lip everts to form a flared aperture on mature adults 37 Rissoa membranacea, .
5: flesh of head and body whitish, translucent, extensively covered by various shades of grey to brown-black pigment dorsally with opaque white spots. As on Atlantic specimens, individuals vary in pigment intensity, and the pattern of relatively darker and lighter areas on individuals is the same 38 Rissoa membranacea.
6: Mediterranean adults with a developed labial varix seem to have less crowded , less randomly arranged , opaque, white spots on their translucent white cephalic tentacles 39 Rissoa membranacea. than those in Orkney. The dots may almost form two distinct lines.
7: opaque, white, dorsal patch behind each eye 38 Rissoa membranacea.
8: foot is translucent white with opaque white spots and pale grey or brown dorsal shading 38 Rissoa membranacea. but some Mediterranean specimens have distinctly yellow soles against which opaque spots show clearly 40 Rissoa membranacea.
9: opercular lobe pale apart from some dark pigment proximally. Pale part often more dirty yellow on Mediterranean specimens than on Atlantic specimens 41 Rissoa membranacea..
10: shallow sublittoral, from 0 m down, usually on various brown algae, mostly Cystoseira sp.
www.mondomarino.net/ricerca/index.asp?view=zoom&p=43&… , occasionally on Posidonia (image M. Mattana flic.kr/p/nPiS2k ) a vascular plant similar in structure to Zostera.
Similar species
Rissoa parva (da Costa, 1788).
Species account at https://marinvert.senckenberg.science/rissoa-parva/
Typical adult R. parva can easily be identified by the brown falciform mark (‘comma’) across the labial varix on adults 42 Rissoa membranacea . The comma is diagnostic when present, but sometimes absent. Then, large Rissoa parva may superficially resemble juvenile R. membranacea if they have similar sinuous costal lines; such as on image 43 Rissoa membranacea .
1: usual maximum height 5mm.
3: tumidity of whorls and presence of ribs vary on both species, but R. parva lacks the distinct subsutural collar of R. membranacea.
5: unpigmented, white head, sometimes with a thin black medial line 43. 43.1 Rissoa membranacea album.
6: single white line, sometimes with breaks, on cephalic tentacles 43. 43.1 Rissoa membranacea album.
7: yellow eye patches 43. 43.1 Rissoa membranacea.
9: opercular lobe entirely pigmented black or dark brown 43. 43.1 Rissoa membranacea
10: misidentification is especially likely if it is on Zostera which is favoured by R. membranacea.
Pusillina inconspicua (Alder, 1844)
Species account at https://marinvert.senckenberg.science/pusillina-inconspicua/ .
1: maximum size varies locally 1.3 to 3 mm.
Tiny purple apical spot confined to single embryonic whorl, 44 Rissoa membranacea .Diagnostic when present, but absent from some.
5: Snout often has broad medial blackish or brown-umber band that extends onto head and body but not onto the side or near the tip of the snout 44 Rissoa membranacea .
6: Cephalic tentacles translucent white with two parallel rows of substantial, opaque-white, hyphen-like marks in each tentacle 44 Rissoa membranacea.
10: this tiny species is often very numerous in company with R. membranacea on Zostera in Britain.
Pusillina sarsii (Lovén, 1846)
Baltic specimens may be almost indistinguishable from R. membranacea unless the radulae are compared (Warén, 1996).
1: maximum height 5mm in Denmark (Rasmussen, 1973)
5: no dark pigment on the side of the snout 45 Rissoa membranacea.
8: foot lacks opaque white spots, and it has a broad, black, transverse band on its dorsal surface that extends onto the edge of the sole 45 Rissoa membranacea (in common with R. parva and P. inconspicua).
10: Often found accompanying R. membranacea on Zostera in Scandinavia.Rissoa guerinii Récluz, 1843
nature22.com/estran22/mollusques/gasteropodes/gasteropode…
From the south coast of England southwards R. guerinii may be confused with ribbed forms of R. membranacea.
3: adapical angle of aperture smoothly rounded (angled, c. 70º, on R. membranacea) 46 Rissoa membranacea.
2: fine spiral ridges distinct where they cross the grooves between the large costal ribs 46 Rissoa membranacea.
10: sometimes accompanying R. membranacea on Zostera, and on other weeds
Distribution and status
Lofoten, Norway to southern Portugal, into Baltic as far as Puck Bay in Poland and Kalmarsund, Sweden. Probably into Mediterranean and Black Sea. GBIF map www.gbif.org/species/5192342
It is difficult to assess the current (2017) position in Britain as many records are of dead shells which may be the remains of extinct populations; most records do not indicate whether live or not NBN UK map species.nbnatlas.org/species/NHMSYS0021055543 . It seems to be commoner in the south of England. Live ones appear to be generally scarce but are locally common on some Zostera beds.
Acknowledgements
I am most grateful to my co-authors, Simon Taylor and Inga Williamson, for providing live specimens for photography, and Alen Petani for finding and photographing specimens. I also thank Jakov Prkić, Fabio Vitale, and Florence and Marc Cochu for their generous help with information and use of their images.
Links and references
Cadée, G.C. 1998. Rissoa membranacea (J. Adams, 1800) (Gastropoda, Prosobranchia) from the Dutch Wadden Sea Basteria, 61, 89 to 98. free pdf at
natuurtijdschriften.nl/search?identifier=597119;keyword=B…
Forbes, E. & Hanley S. 1849-53. A history of the British mollusca and their shells. vol. 3 (1853), London, van Voorst. Free pdf at archive.org/stream/historyofbritish03forbe#page/98/mode/2up
Use slide at base of page to select pp. 98 – 103 .
Fretter, V. and Graham, A. 1962. British prosobranch molluscs. London, Ray Society.
Fretter, V. and Graham, A. 1978. The prosobranch molluscs of Britain and Denmark. Part 4– Marine Rissoacea. J. Moll. Stud. Suppl. 6, 153-241
Fretter, V. and Pilkington, M.C. 1970. Prosobranchia. Veliger larvae of Taenioglossa and Stenoglossa. Conseil international pour l’exploration de la mer. Fiches d’identification. Zooplankton, 129-132. PDF
Goor, A.C.J. van, 1919. Het zeegras (Zostera marina L.) en zijn beteekenis voor het leven der visschen. Rapp. Verh. Rijks. Inst. Visscherijonderz. 1(4): 415 to 498.
Graham, A. 1988. Molluscs: prosobranch and pyramidellid gastropods: keys and notes for the identification of the species. Brill & Backhuys, for Linn. Soc. Lond. & Estuarine and Brackish-water Sciences Assoc. Synopses of the British Fauna (New Series) no.2. Edition 2 (662pages). Leiden. (Edition 1 of series, 1971, 112 pages, is no substitute.) See Wigham (2017) below for revised edition.
Heide,T. van der & Katwijk, M.M. van & Geerling, G.W. 2006. Een verkenning van de groeimogelijkheden van ondergedoken Groot zeegras (Zostera marina) in de Nederlandse Waddenzee. Rijksinstituut voor Kust en Zee. Free pdf at publicaties.minienm.nl/documenten/een-verkenning-van-de-g…
Jeffreys, J.G. 1862-69. British conchology. vol. 4 (1867). London, van Voorst. Free pdf at archive.org/stream/britishconcholog04jeffr#page/30/mode/2up . Use slide at base of page to select pp. 30 to 32 .
Petersen, C.G.J. 1918. The sea bottom and its production of fish-food. Rep. Danish Biol. Sta., 25, 1 to 62.
Rasmussen, E. 1973. Systematics and ecology of the Isefjord marine fauna (Denmark). Ophelia, 11, 1-495.
Rehfeldt, N. 1968. Reproductive and morphological variations in the prosobranchRissoa membranacea. Ophelia 5: 157 to 173.
Verduin, A. 1982. On the taxonomy and variability of Recent European and North African marine species of the subgenus Rissostomia Sars, 1878, of the genus Rissoa Desmarest, 1814 (Mollusca, Gastropoda, Prosobranchia). Basteria, 45, 143 to 166. free pdf at natuurtijdschriften.nl/search?identifier=596749;keyword=B…
Warén, A. 1996. Ecology and systematics of the north European species of Rissoa and Pusillina (Prosobranchia: Rissoidae). J. mar. biol. Ass. U.K. 76, 1013-1059.
Wigham, G.D. 1975a. The biology and ecology of Rissoa parva (da Costa). [Gastropoda: Prosobranchia]. J. mar. biol. Ass. U.K. 55: 45-67.
Wigham, G.D. 1975b. Environmental influences upon the expression of shell form in Rissoa parva (da Costa). [Gastropoda: Prosobranchia]. J. mar. biol. Ass. U.K. 55: 425-438.
Abstract at journals.cambridge.org/action/displayAbstract?fromPage=on…
Wigham, G.D. & Graham, A. 2017. Marine gastropods 2: Littorinimorpha and other, unassigned, Caenogastropoda. Synopses of the British Fauna (New Series) no.61. (344 pages). Field Studies Council,Telford, England.
Current taxonomy: World Register of Marine Species (WoRMS)
www.marinespecies.org/aphia.php?p=taxdetails&id=141359
Glossary
adapical = towards the apex of the shell.
adelphophagy = consumption by one larva of non-developing ‘nurse’ eggs in same egg capsule.
adelphophagous = (adj.) practising adelphophagy.
aperture = mouth of gastropod shell; outlet for head and foot.
Atlantic = (for purposes of this account) the Atlantic and its various branches such as the Baltic and North Seas, but not the Mediterranean.
auct. = (abbreviation of “auctorum” = “of authors”) name, often of another valid species, used in error for this one by other author(s). en.wikipedia.org/wiki/Auctorum
bifid = divided into two parts by a cleft.
cephalic = (adj.) of or on the head.
coll. = (or “in coll.”, abbreviation of “in collectionem”) in the collection of (named person or institution; compare with leg.).
columella = solid or hollow axial “little column” around which gastropod shell spirals; hidden inside shell, except on final whorl next to lower part of inner lip of aperture where hollow ones may end in an umbilicus or siphonal canal.
columellar = (adj.) of or near central axis of spiral gastropod.
columellar lip = lower (abapical) part of inner lip of aperture.
costa = strong rib running across a whorl of a gastropod shell at right angles to direction of coiling and any spiral striae.
costae = (pl.) strong ribs running across a whorl of a gastropod shell at right angles to direction of coiling and any spiral striae.
costal = (adj.) of, or arranged like, costae.
costate = bearing costae.
diatom = microscopic aquatic alga with siliceous cell-walls.
ctenidium = comb-like molluscan gill; usually an axis with a row of filaments either side.
distal = away from centre of body or from point of attachment.
ELWS = extreme low water spring tide (usually near March and September equinoxes).
height = (of gastropod shells) distance from apex of spire to base of aperture.
in coll. = (abbreviation of in collectionem) in the collection of (named person or institution; compare with leg.).
in litt. = (abbreviation of Latin “in litteris”) in upublished correspondence.
labial varix = especially strong or broad costa (rib) along edge of outer lip of aperture. Sometimes other varices mark positions of previous prolonged pauses in growth.
lecithotrophic = feeding on yolk of own egg, and, in the case of several marine gastropods, the yolks of non-developing ‘nurse’ eggs in the same egg capsule. Larvae feeding in this manner develop large larval shells which become large protoconchs on the adults.
leg. = (abbreviation of legit) ‘he/she collected’ i.e. collected/ found by (compare with in coll.)
mantle = sheet of tissue that secretes the shell and forms a cavity for the gill in most marine molluscs.
Mediterranean = (for purposes of this account) the Mediterranean and its branches such as the Adriatic Sea.
metapodium = hind part of the foot.
microphytes = microscopic unicellular algae, including diatoms.
mucus = (noun) viscous, slippery substance secreted by various glands on molluscs.
mucous [also mucal, mucosal] = (adj.) pertaining to mucus.
opercular = (adj.) of the operculum.
opercular disc = part of foot that growing operculum rotates on.
opercular lobe = extension of opercular disc beyond edge of operculum.
operculum = plate of horny conchiolin, rarely calcareous, used to close shell aperture.
opisthocline = (of costae, growth lines etc) adapical end tilted back towards earlier growth (to right on apertural view of dextral gastropod).
orthocline = (of costae, growth lines etc) parallel to axis of shell, not tilted.
peristome = entire rim of aperture.
plankton = animals and plants that drift in pelagic zone (main body of water).
planktotrophic = feeding on plankton. (Veliger larvae feeding in this manner develop small larval shells which become small protoconchs on the adults.)
prosocline = (of costae, growth lines etc) adapical end tilted forwards towards more recent growth (to left on apertural view of dextral gastropod).
proximal = towards the centre of the body or point of attachment.
stria = (pl. striae) very narrow spiral groove.
suture = groove or line where whorls of gastropod shell adjoin.
umbilicus = cavity up axis of some gastropods, open as a hole or chink on base of shell, often sealed over.
veliger = shelled larva of marine gastropod or bivalve mollusc which swims by beating cilia of a velum (bilobed flap).
WoRMS = World Register of Marine Species, www.marinespecies.org/index.php