Click image to enlarge with full caption. Main text below slider.
Patella depressa Pennant, 1777
PDF version at www.researchgate.net/profile/Ian_Smith19/research
Current taxonomy: World Register of Marine Species (WoRMS)
www.marinespecies.org/aphia.php?p=taxdetails&id=151374
Synonyms: Patella intermedia Murray in Knapp,1857; Patella vulgata var. intermedia Jeffreys, 1865; Patella athletica [as dark variant of] Forbes & Hanley,1849;
Jeffreys (1865) mistakenly took the rudimentary description of P. depressa by Pennant (1777) to be what is currently (2015) called P. ulyssiponensis Gmelin, 1791 (syn. P. athletica). Until 1923, most authors followed Jeffreys in applying the name P. depressa Pennant to the wrong species, and in using the name P. intermedia Jeffreys for what is now recognised as P. depressa Pennant. Examination of Pennant’s type specimen by Tomlin (1923) exposed the error and authors started to use the name P. athletica Bean, 1844, for what is now called P. ulyssiponensis but, probably to avoid confusion, many retained use of P. intermedia Jeffreys for the true P. depressa Pennant, despite Pennant’s priority, until the 1970s (e.g.Yonge & Thompson, 1976).
Vernacular names: Black footed limpet (English); Brenigen dorddu (Welsh); Platte schaalhoren (Dutch); Patelle bernique (French);
Meaning of name:
Patella (Latin) = little pan. depressa (Latin) = depressed /low.
Terms in text used with restricted or specialised meaning are marked with hashtag#; refer to GLOSSARY below.
Shell Description
Patellid limpets have great geographical variation within and between species. This account refers to typical British specimens.
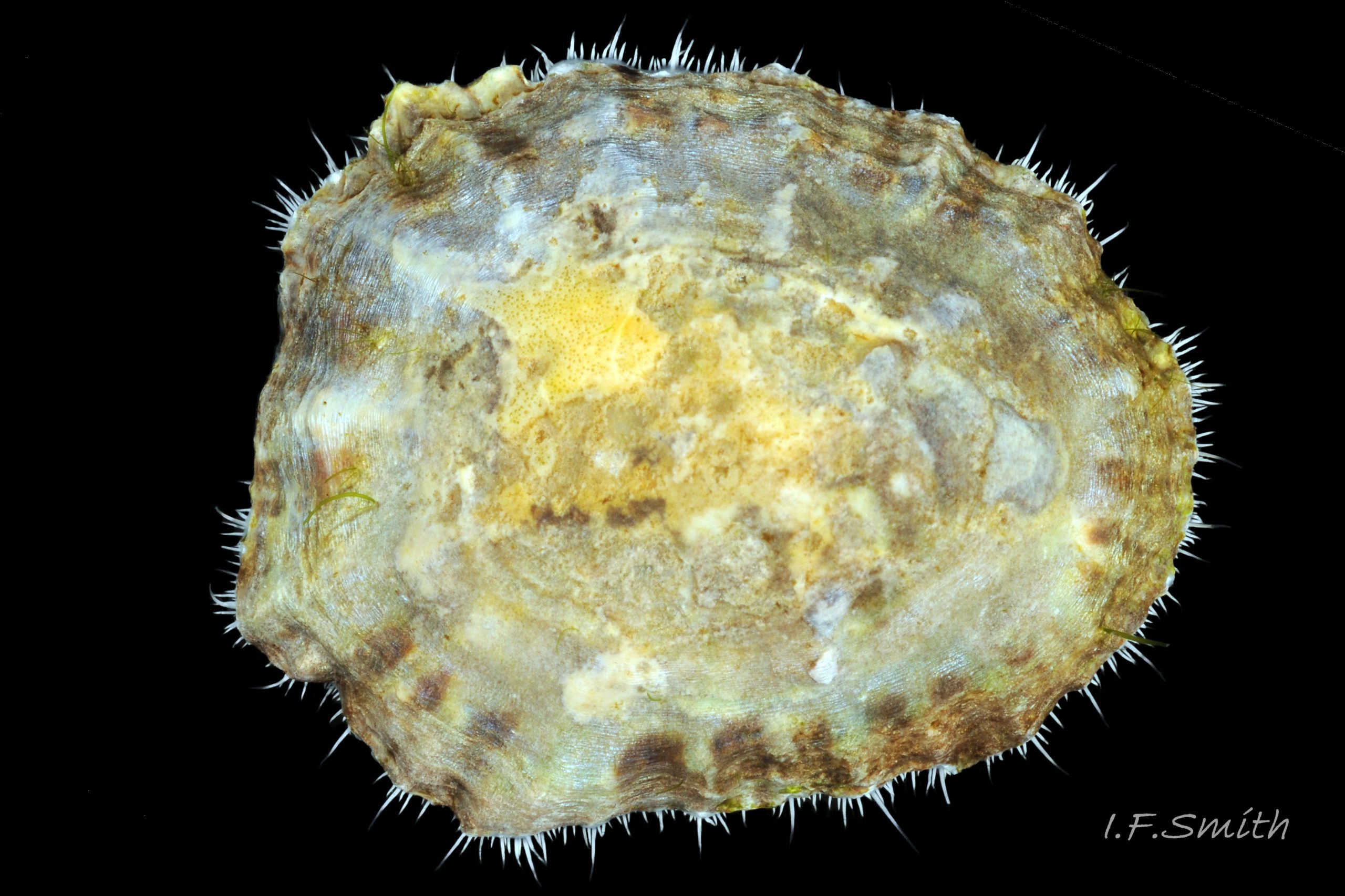
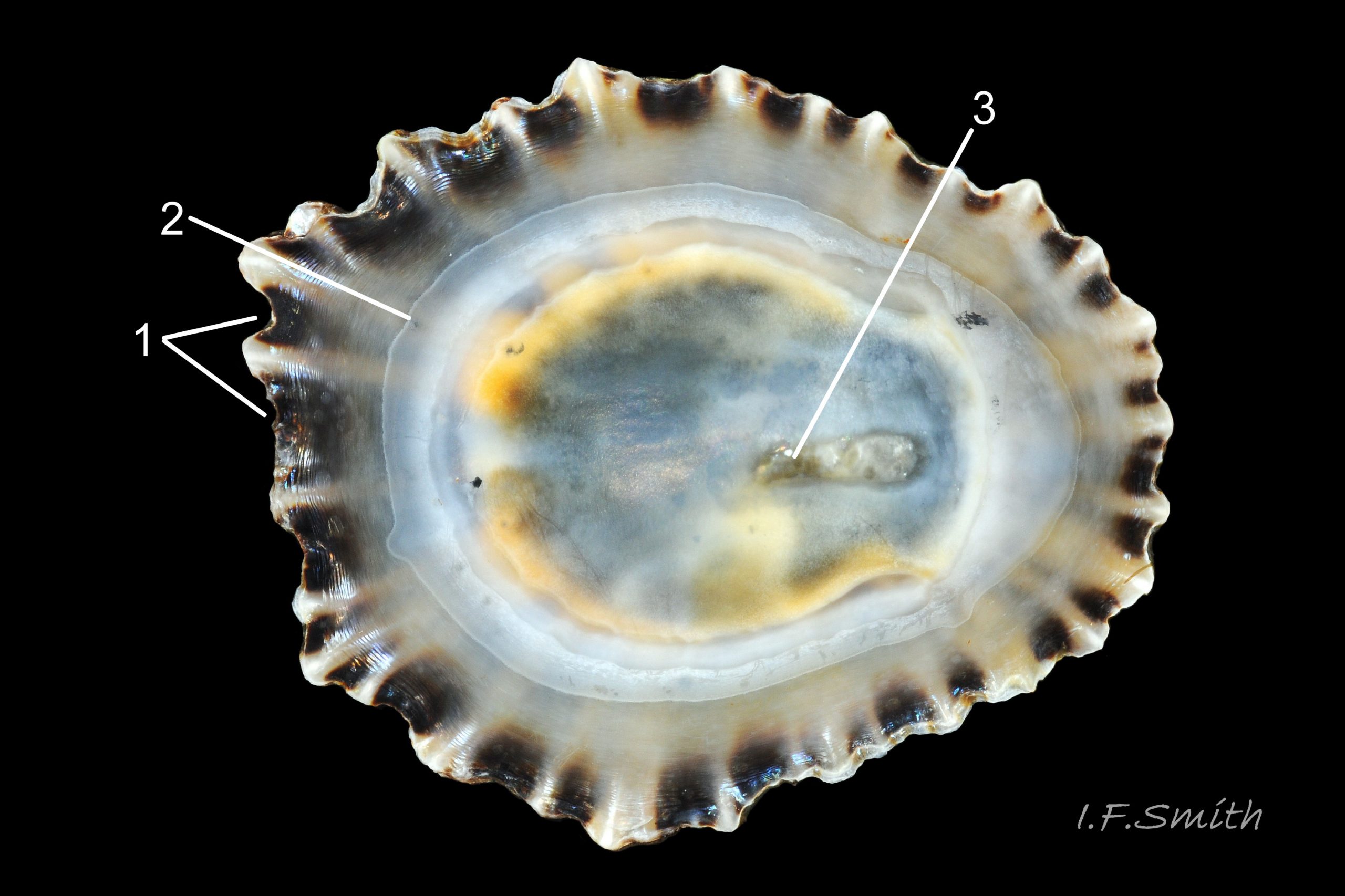
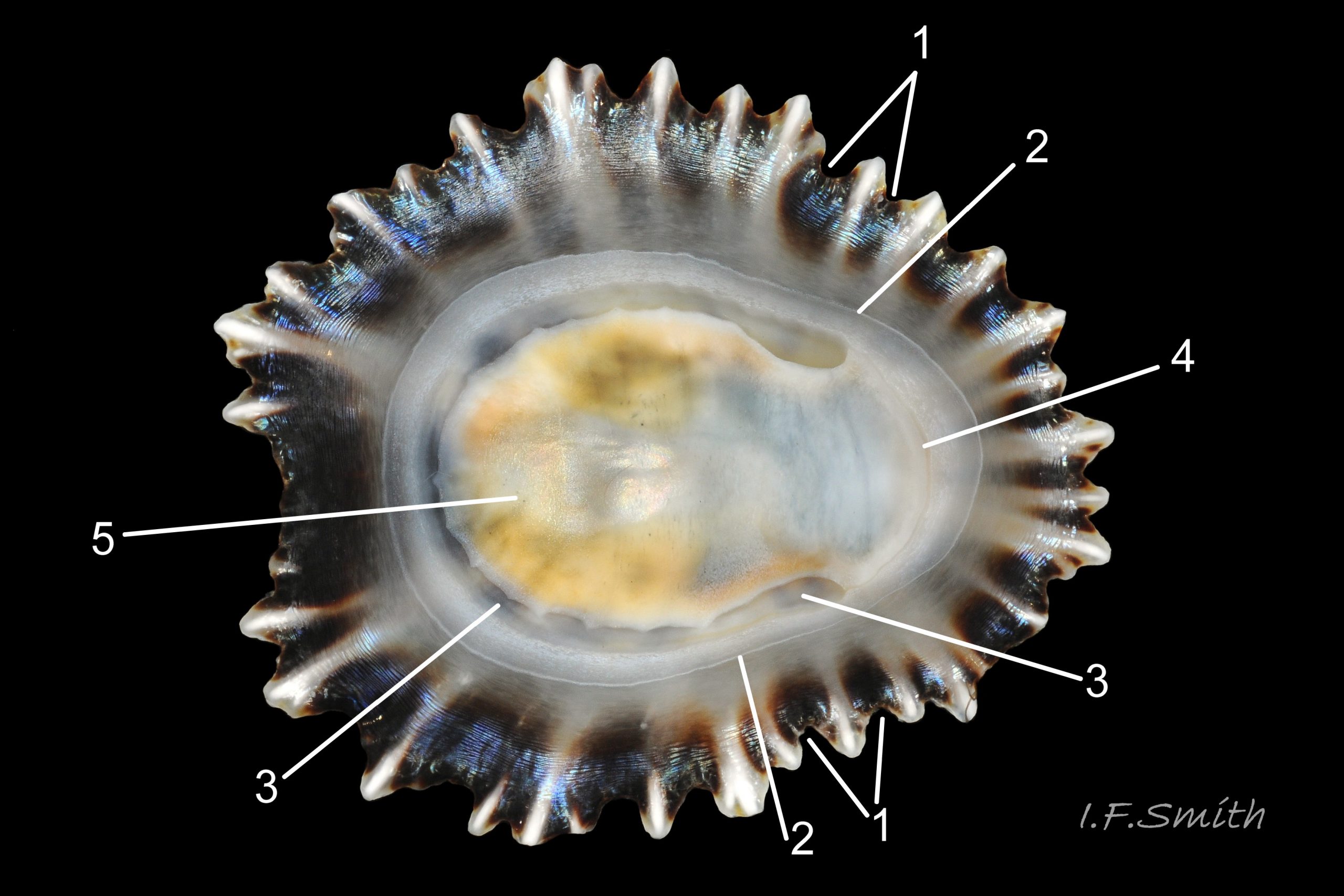
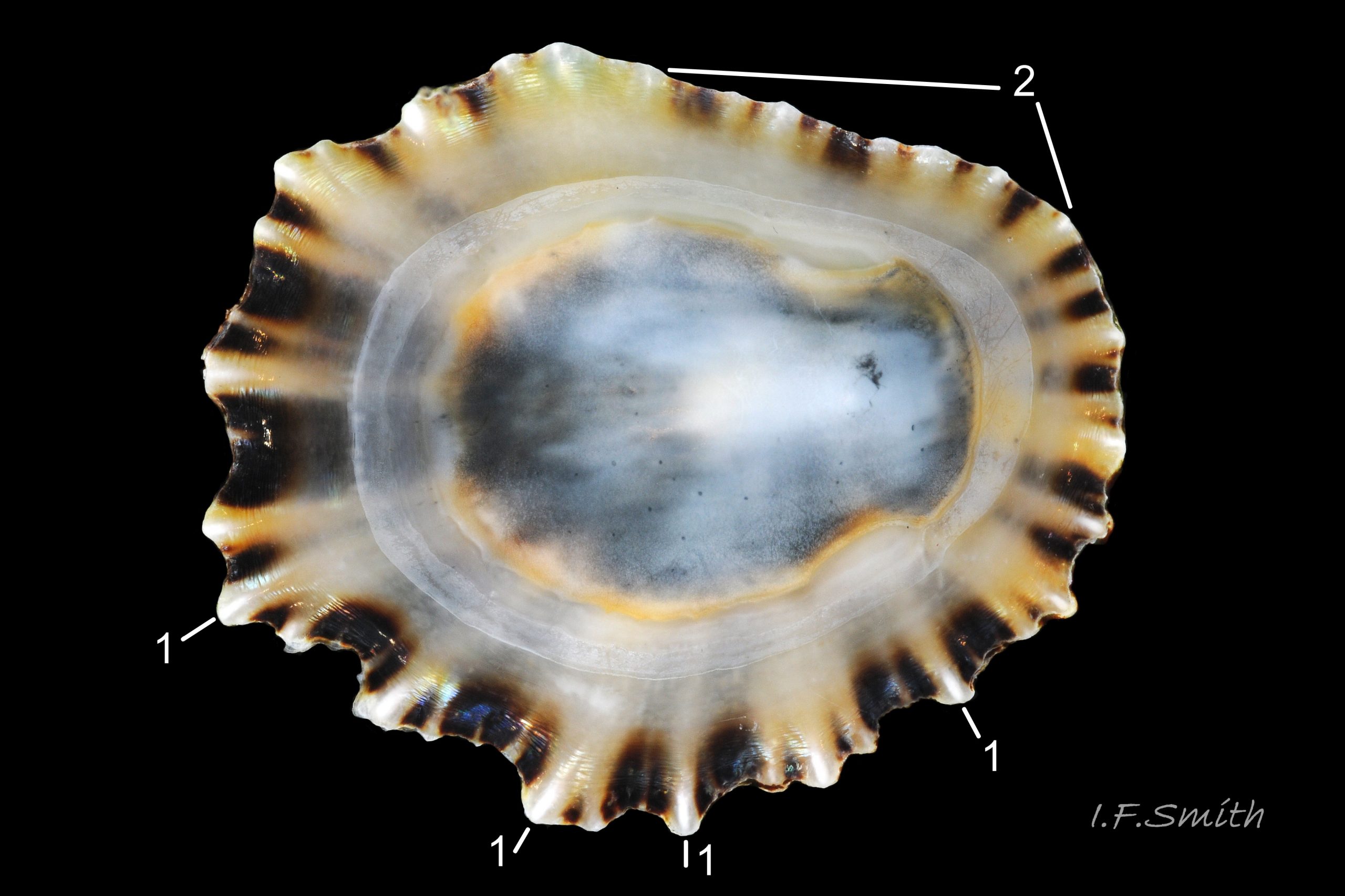
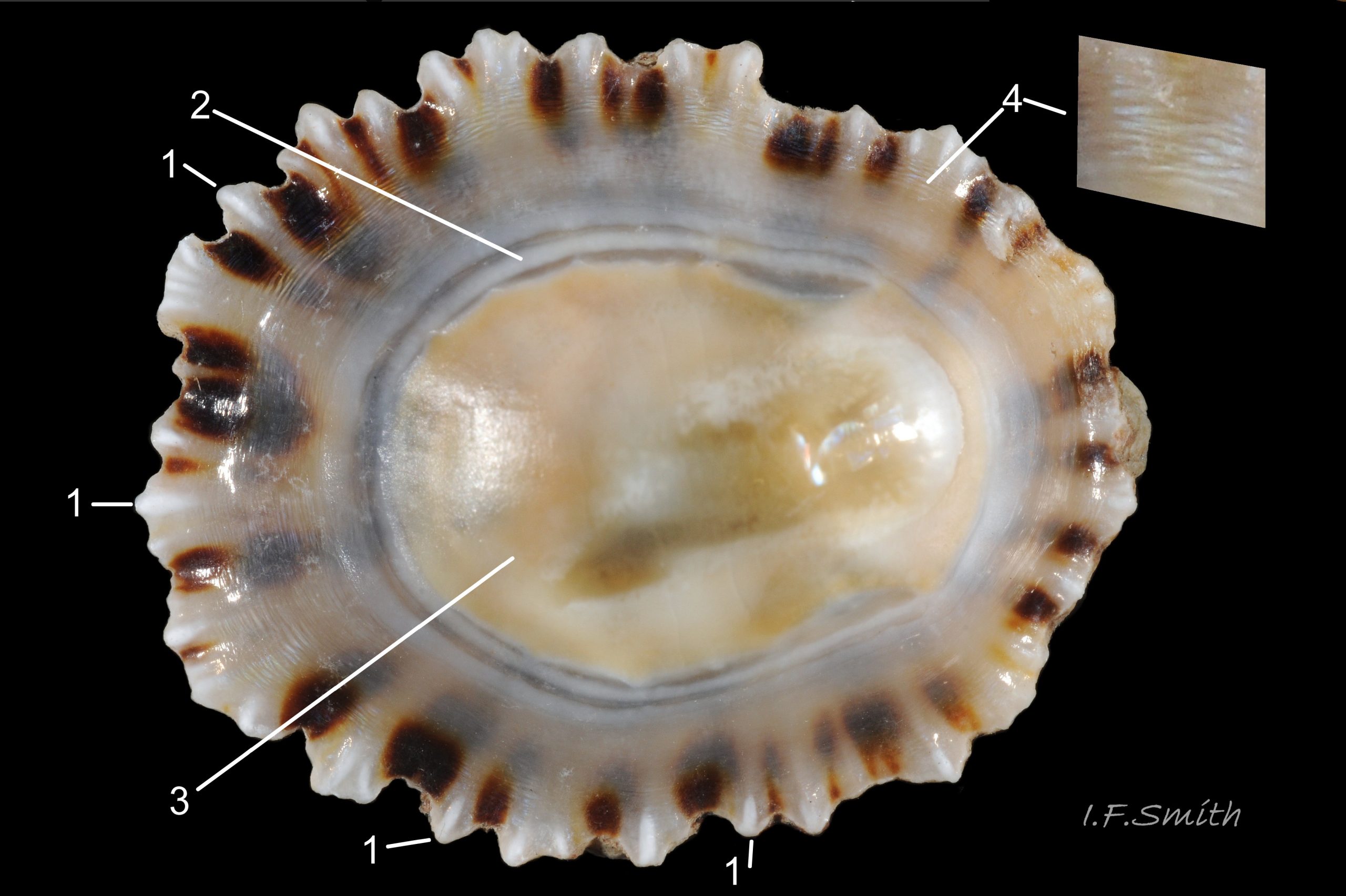
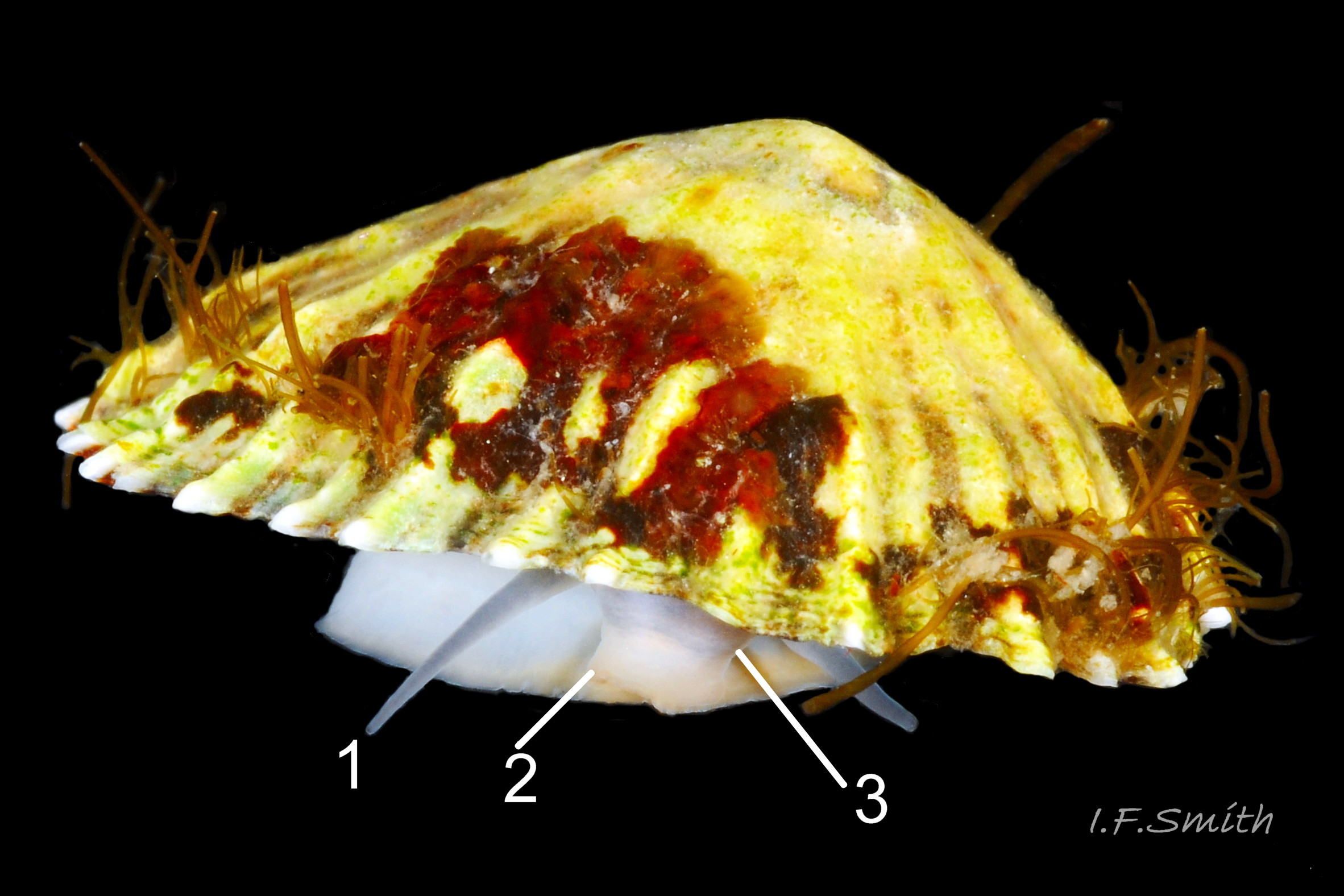
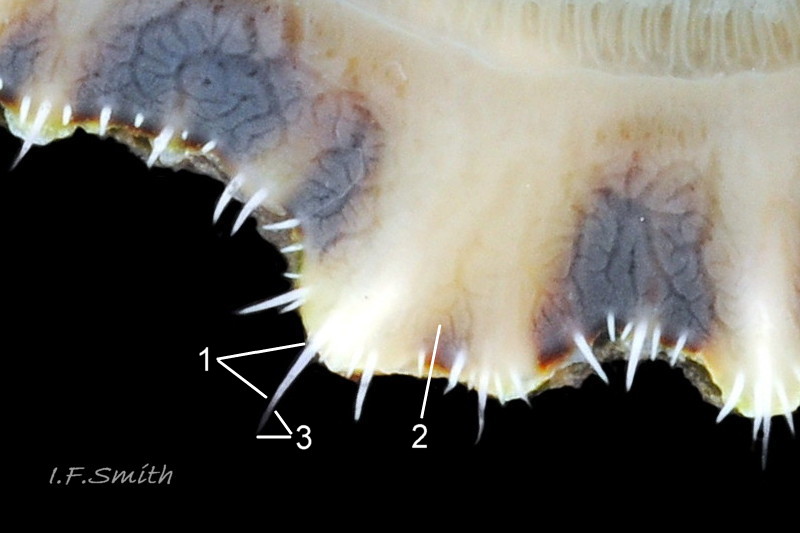
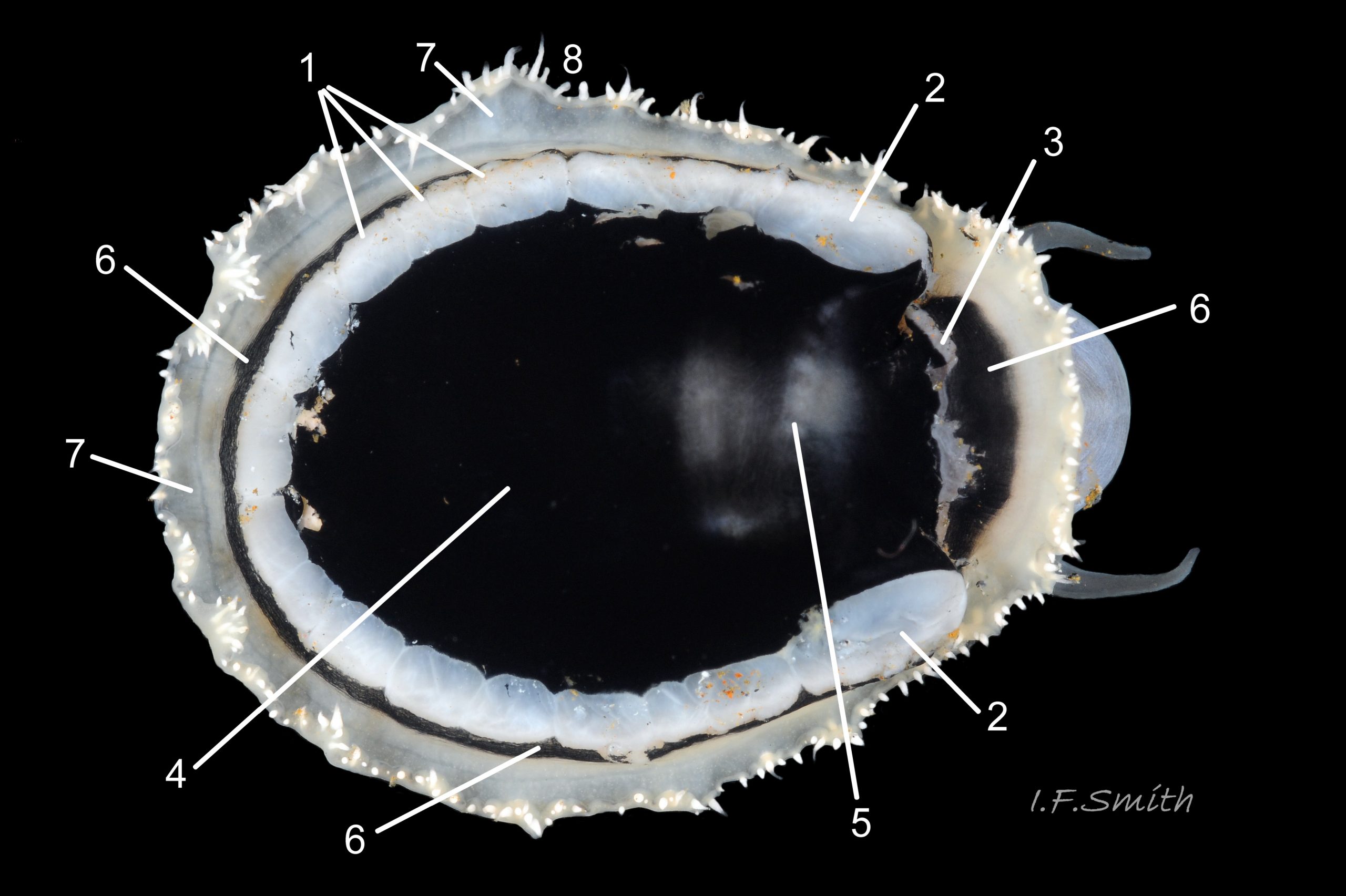
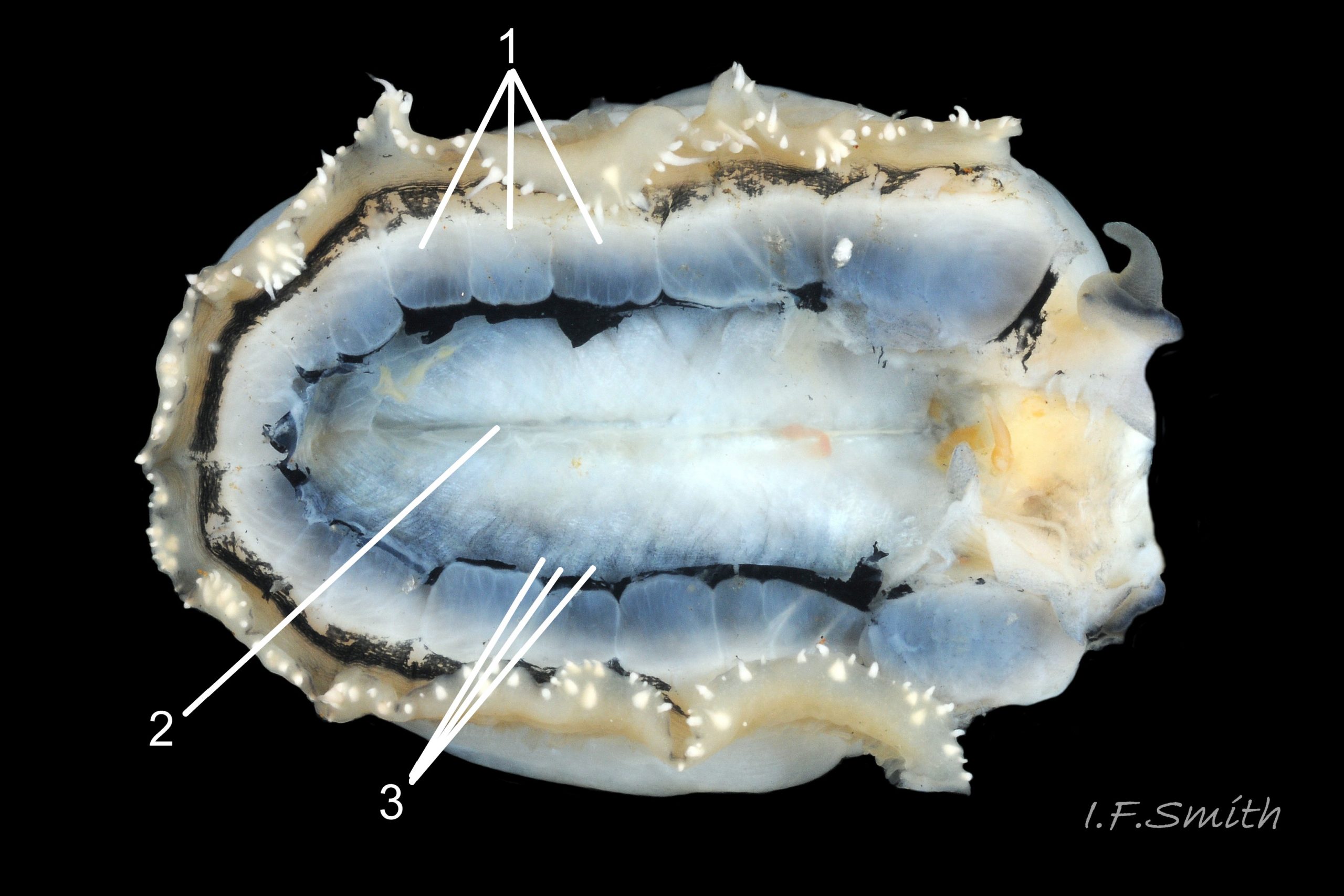
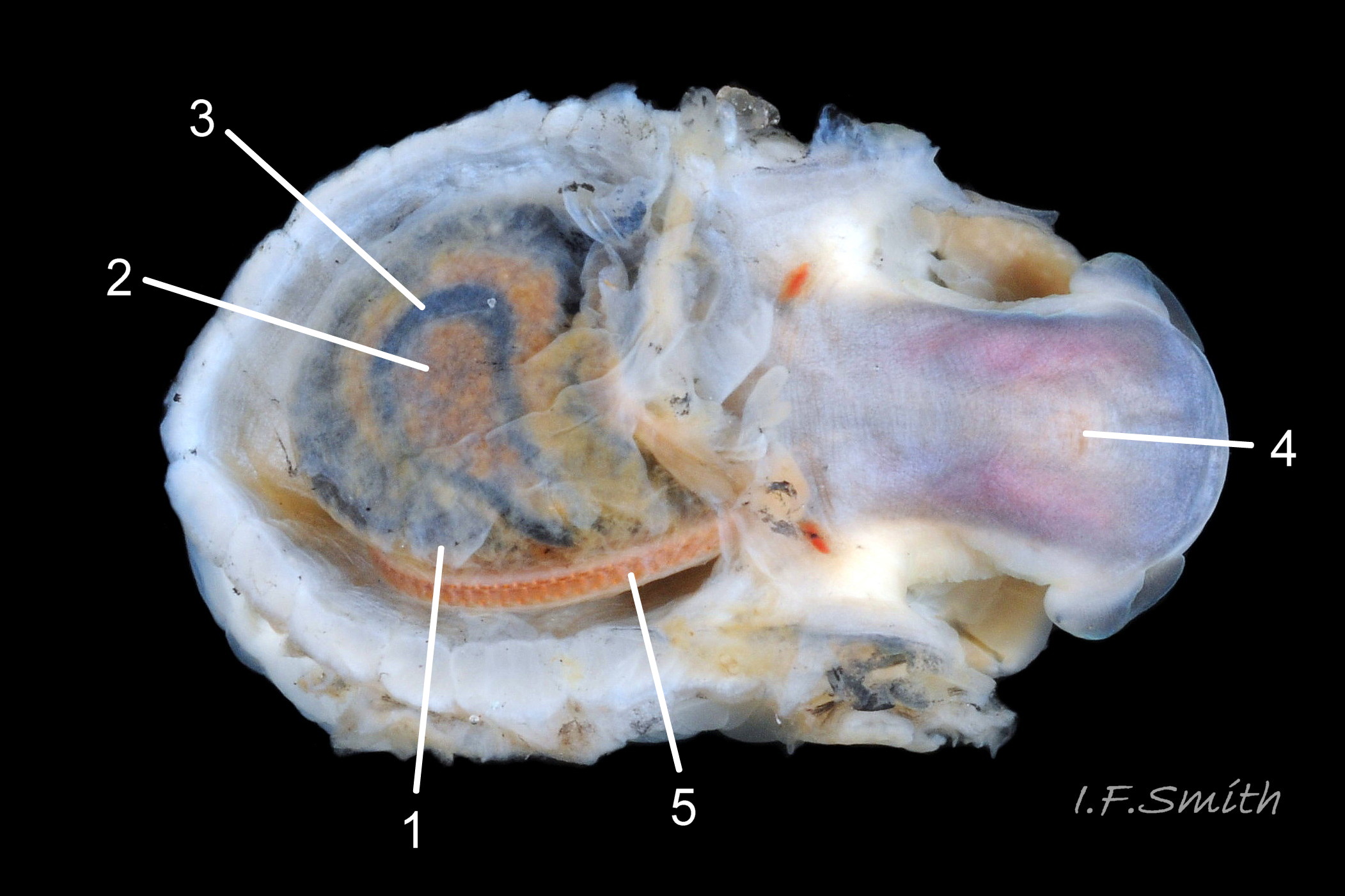
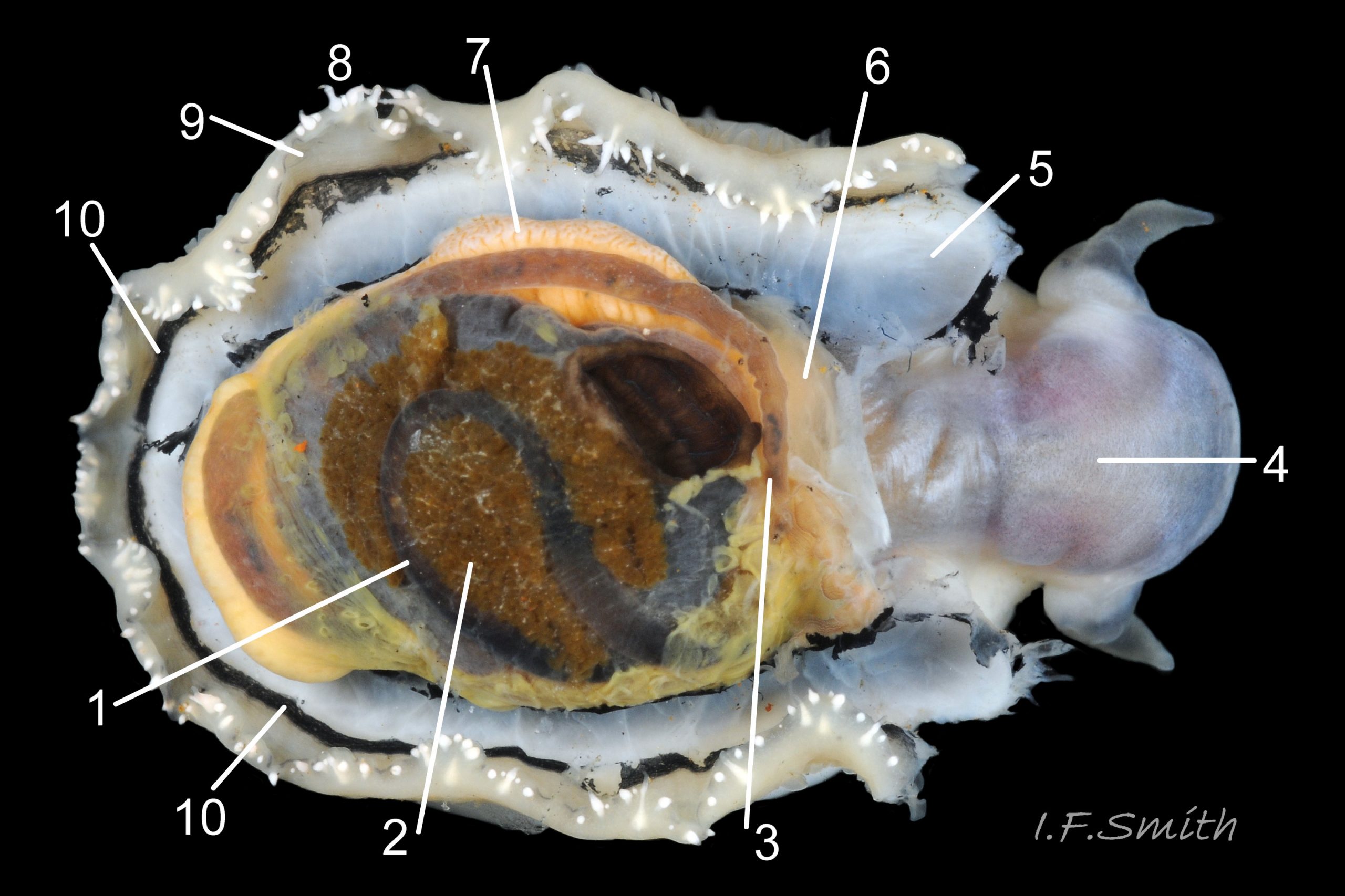
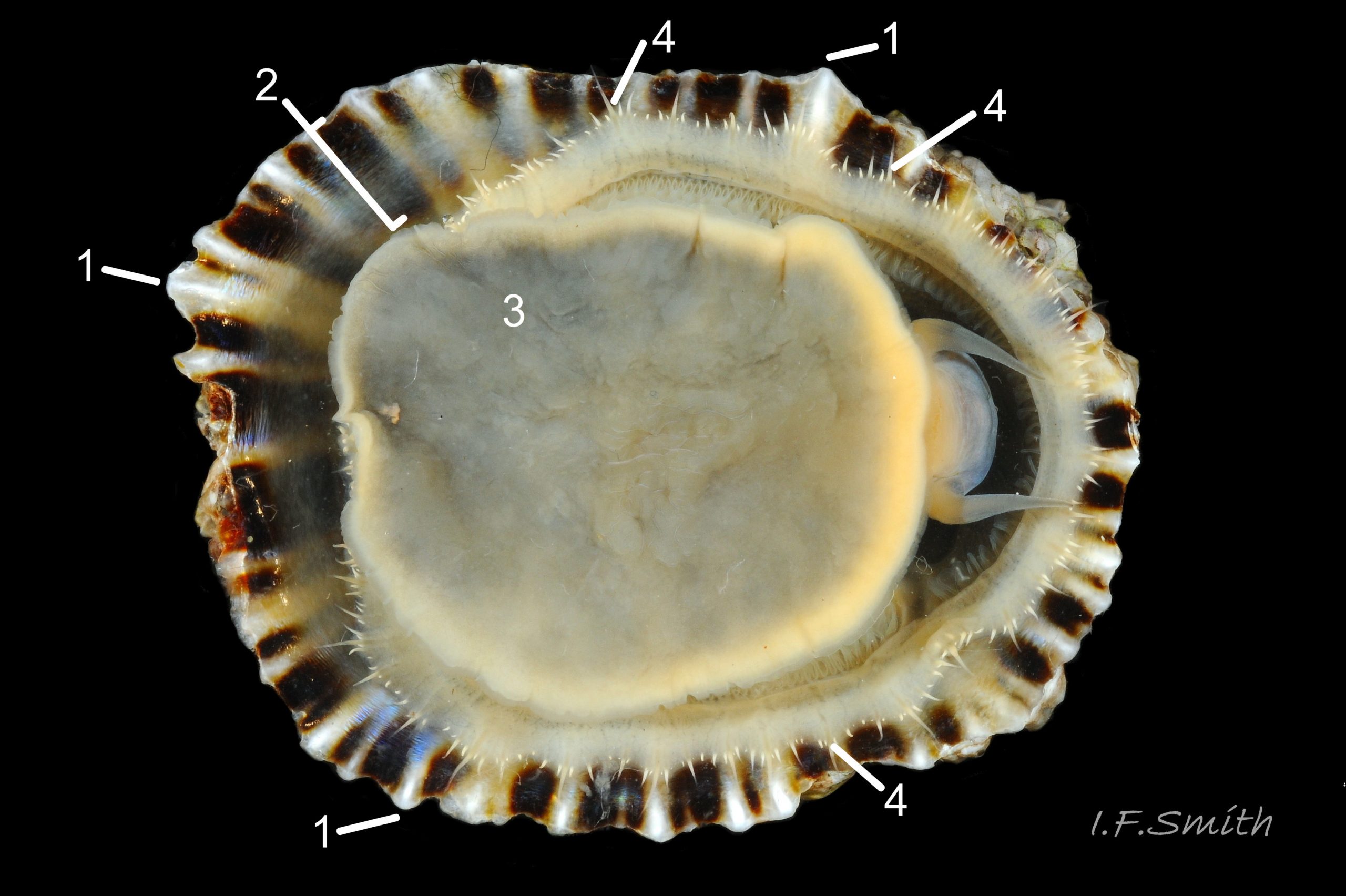
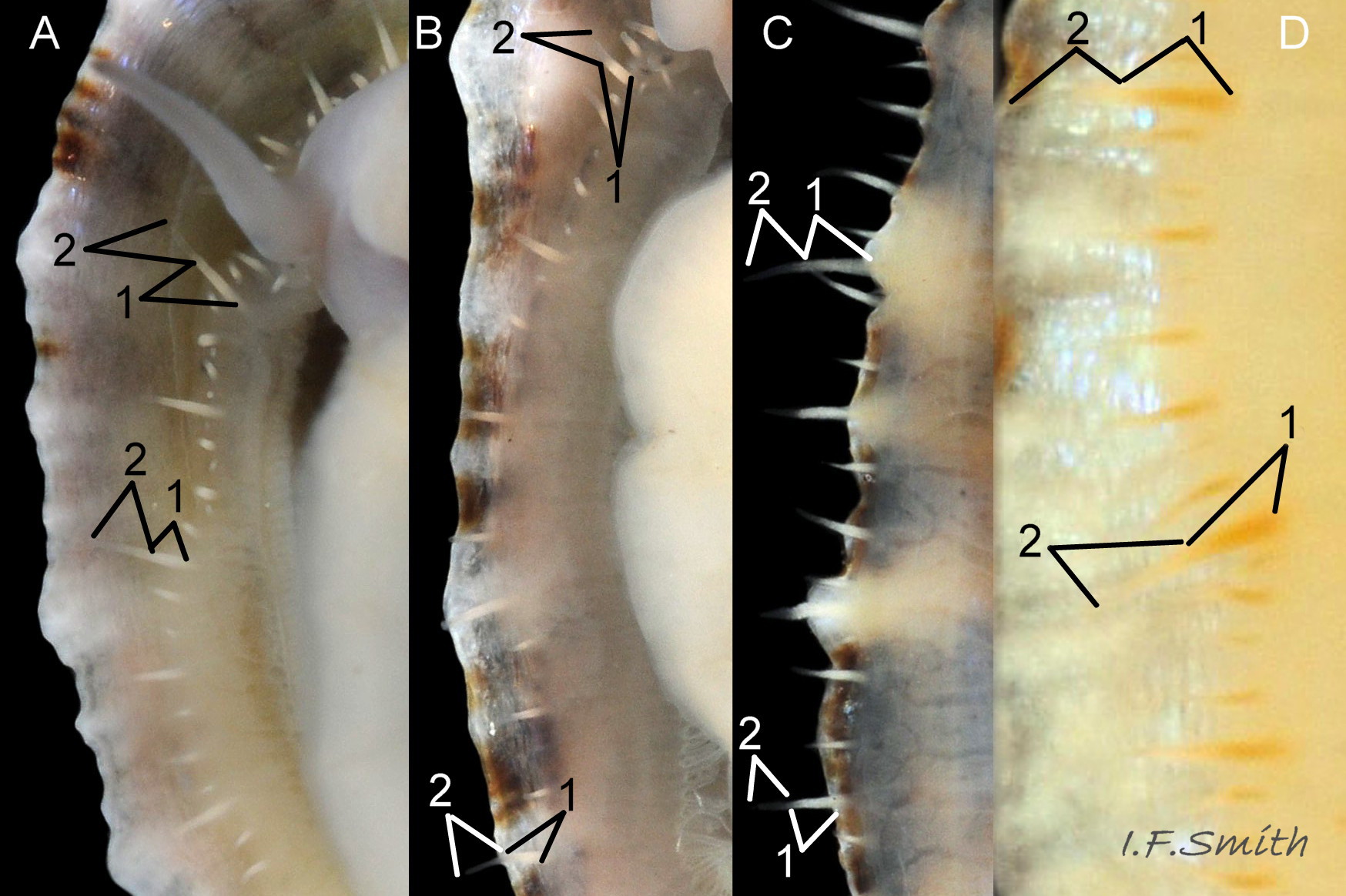
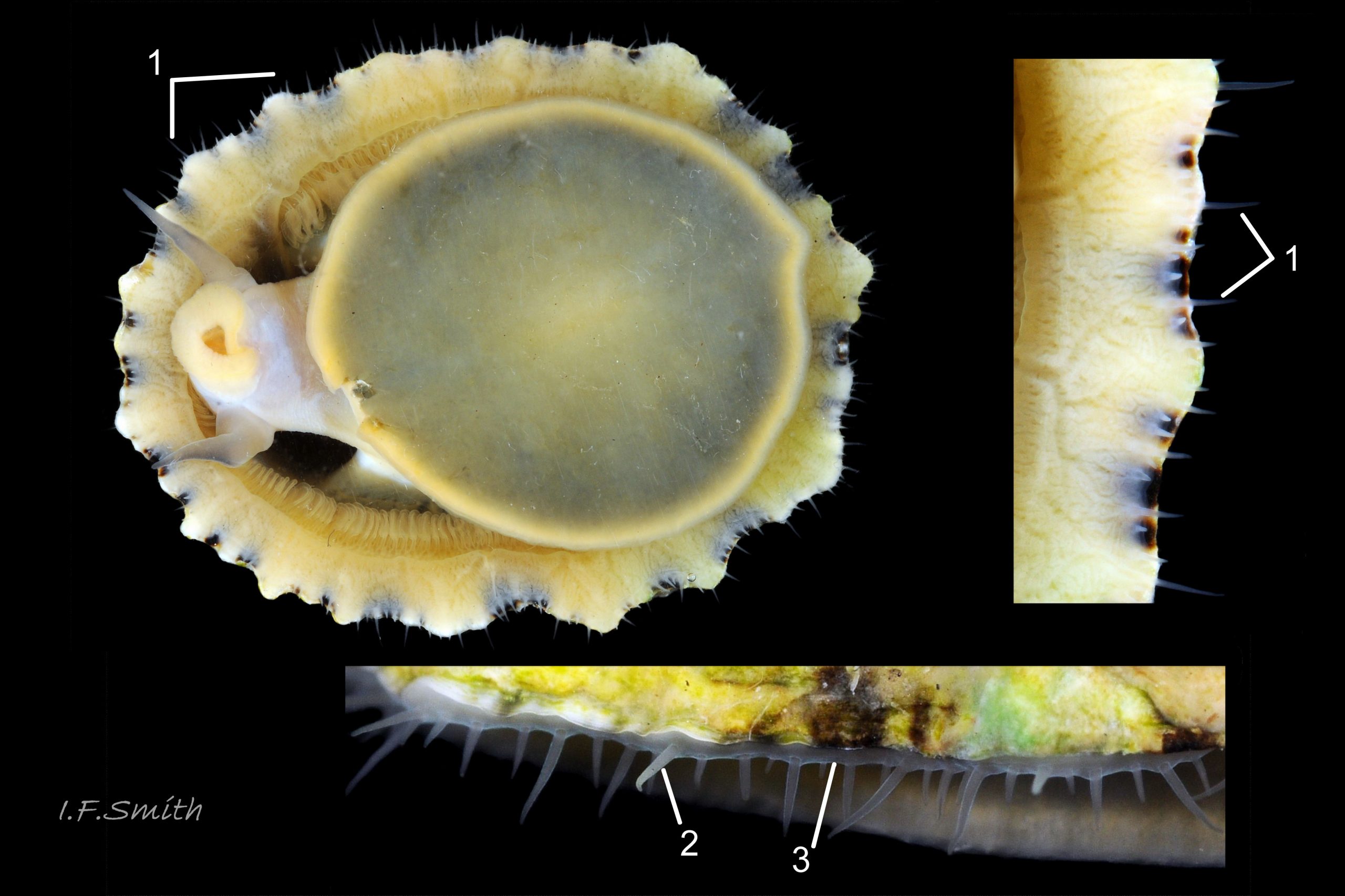
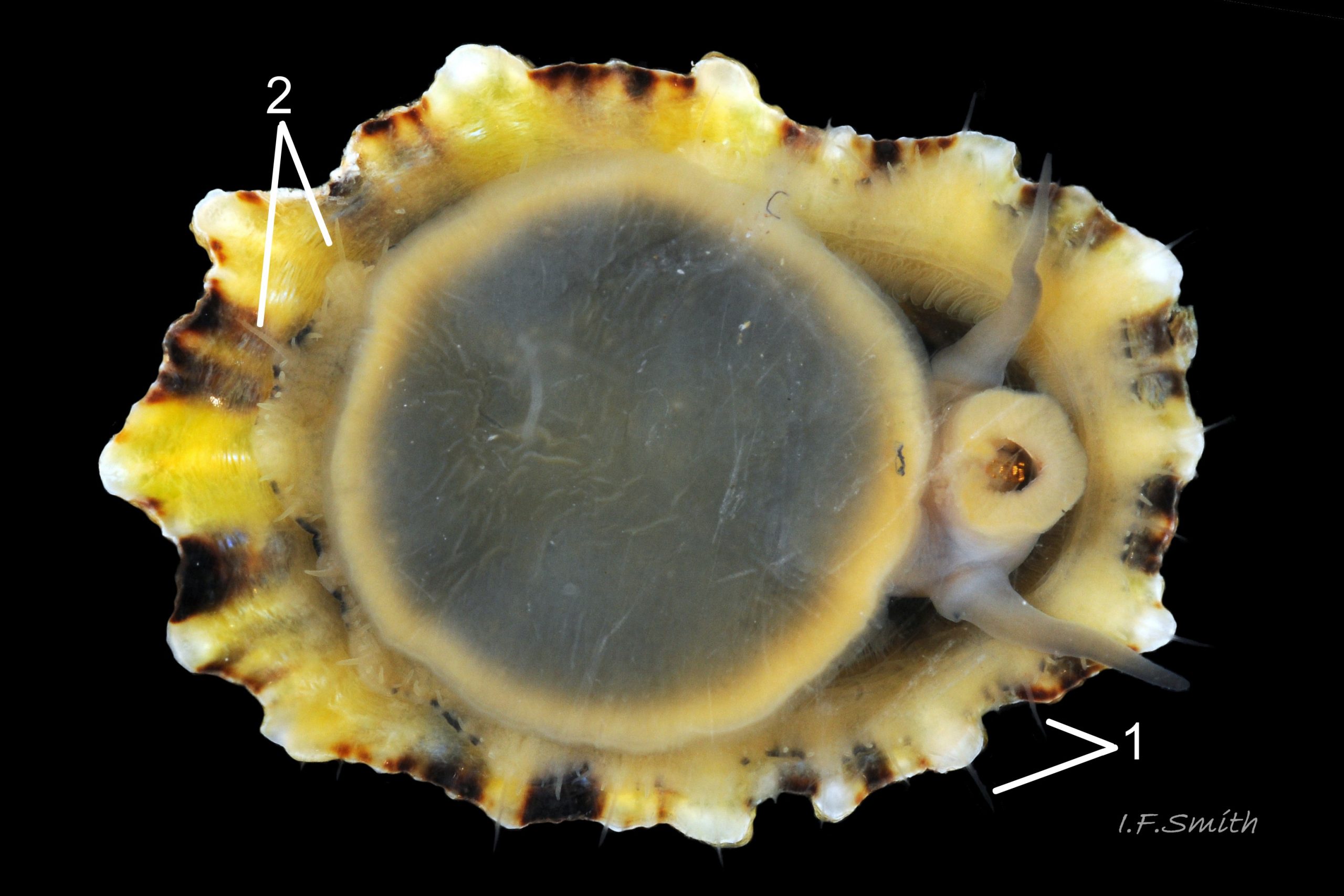
Usual maximum length c.35mm and height# 12mm 01 Patella depressa. Strong. Conoid; apex is positioned to anterior of centre. The base is ovoid , widest and sometimes angulated at posterior. Profile usually low (H/L 25-37% in sample of twelve typical shells from S.W.England and N.W. Wales) 02 Patella depressa. In profile, anterior and posterior usually slightly convex or almost straight. Sculpture of narrow, radiating, whitish ribs that tend to be arranged in triplets; one major flanked by a minor each side 10 Patella depressa. Ribs project as points from aperture-rim of unworn shells 03 Patella depressa, but often majority have ribs rounded, and rib-points reduced, by erosion, and outer shell-layer eroded away from apical half of the shell 04 Patella depressa to almost whole shell 05 Patella depressa. Dark radiating rays in grooves of exterior shell-layer visible externally only on uneroded parts 04 Patella depressa, but usually clearly visible on interior through transparent, iridescent skirt-shell-layer# for much 06 Patella depressa or most 07 Patella depressa of the way to opaque, whitish, pallial-groove-band#. On interior, whitish projecting points of ribs have short, unglazed, chalky-white central line, but reduced or lacking where projecting point of rib eroded 08 Patella depressa. Pedal-retractor muscle leaves translucent horseshoe-shape scar 07 Patella depressa , often containing an opaque white line 09 Patella depressa. Mouth of horseshoe-scar is closed by thin anterior mantle-attachment scar 09 Patella depressa; the two scars enclose area shaped like fat amphora# filled with blackish 10 Patella depressa, grey 11 Patella depressa, orange-cream 12 Patella depressa, opaque-white and/or yellowish-cream 07 Patella depressa shell-layer. Some have excavated, colourless, translucent patch near vertex#, 13 Patella depressa, perhaps caused by re-absorption of shell-material. Juvenile spat lack ridges on main antero-posterior axis and have broad, prominent, mid-lateral pigment lines from apex to lip that are swept forwards on left and backwards on right.
Body description
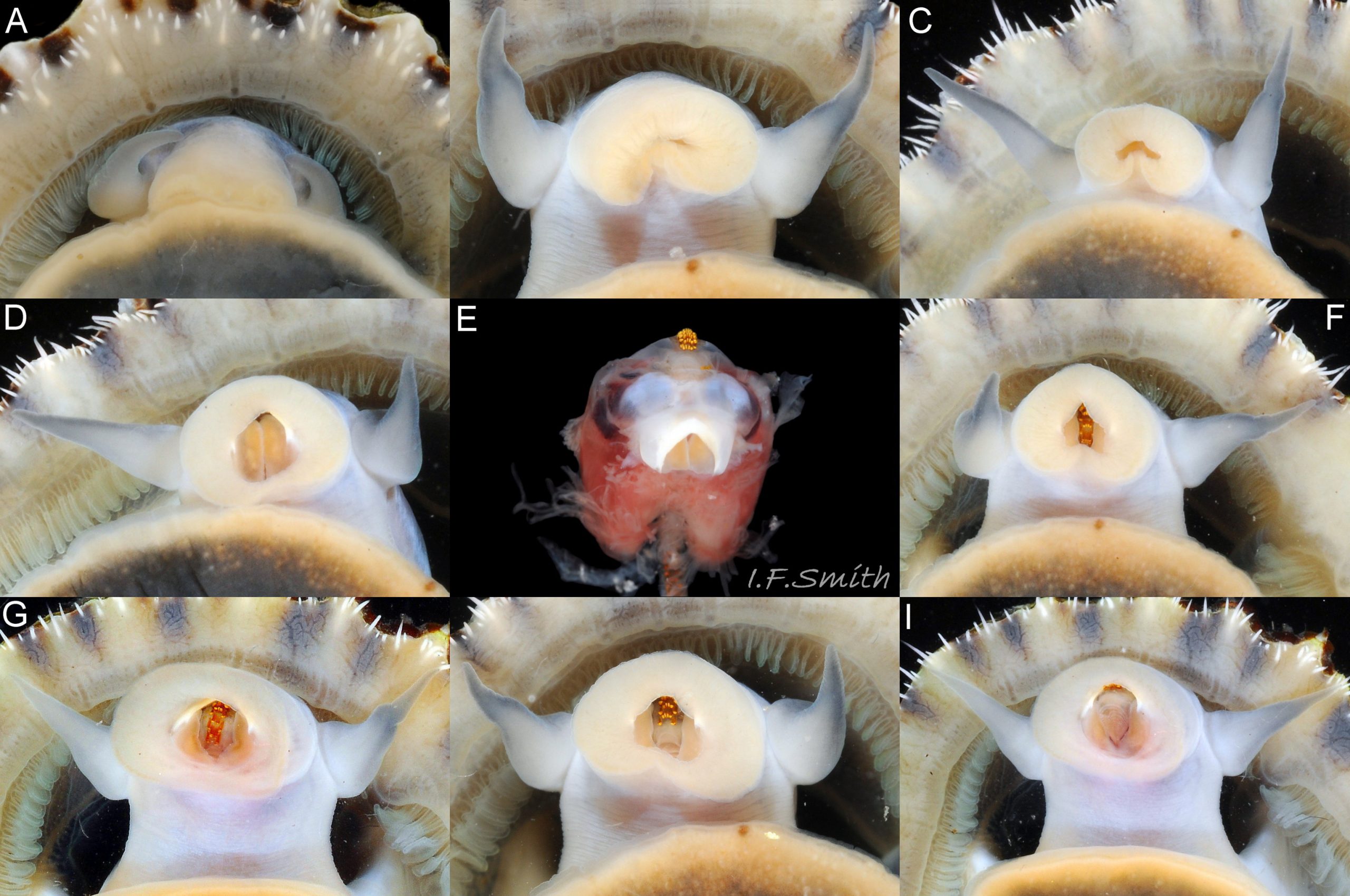
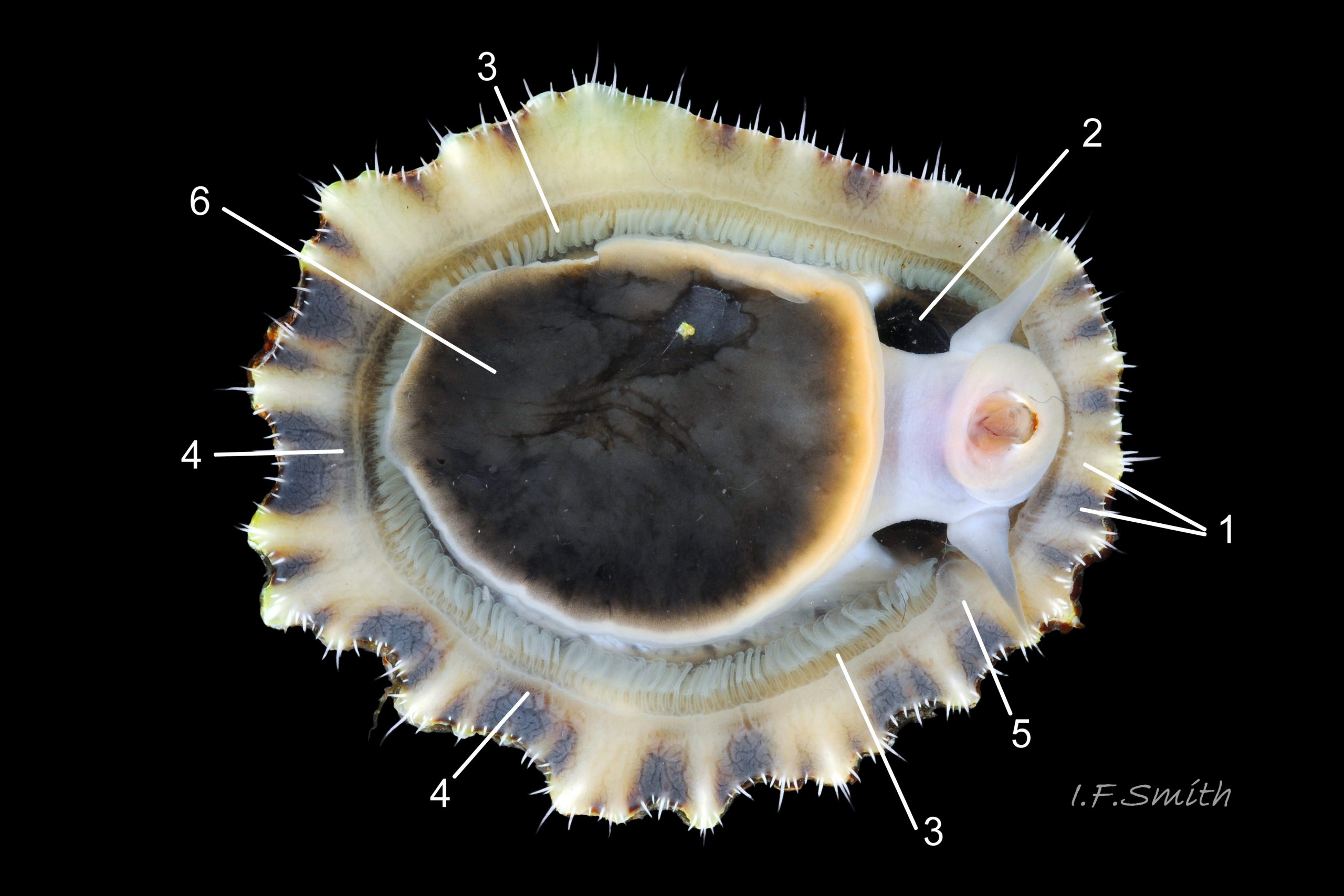
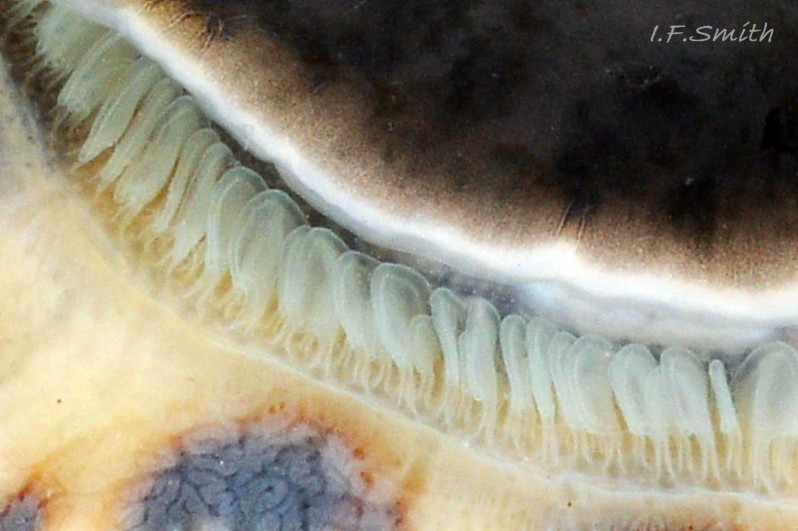
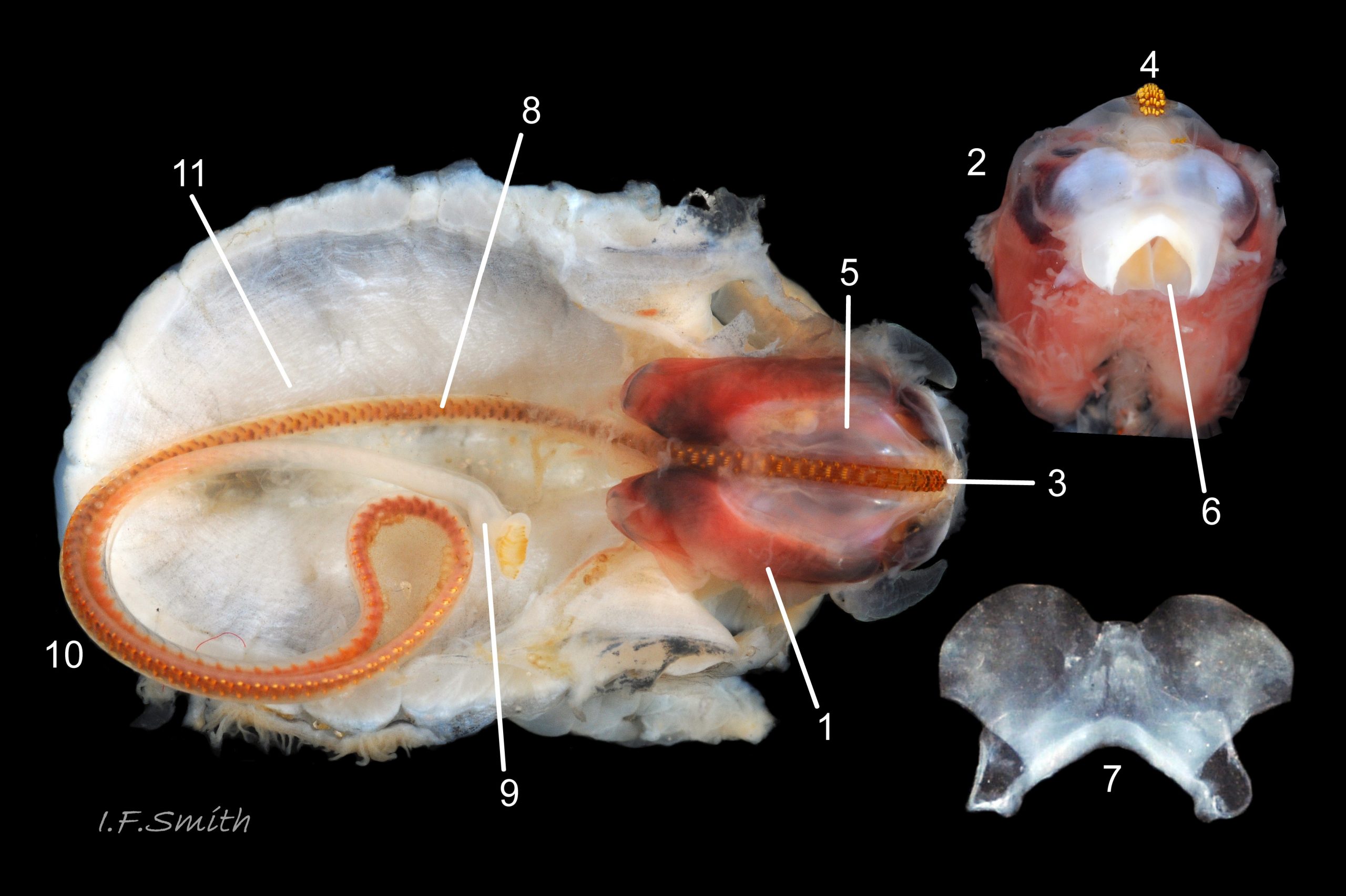
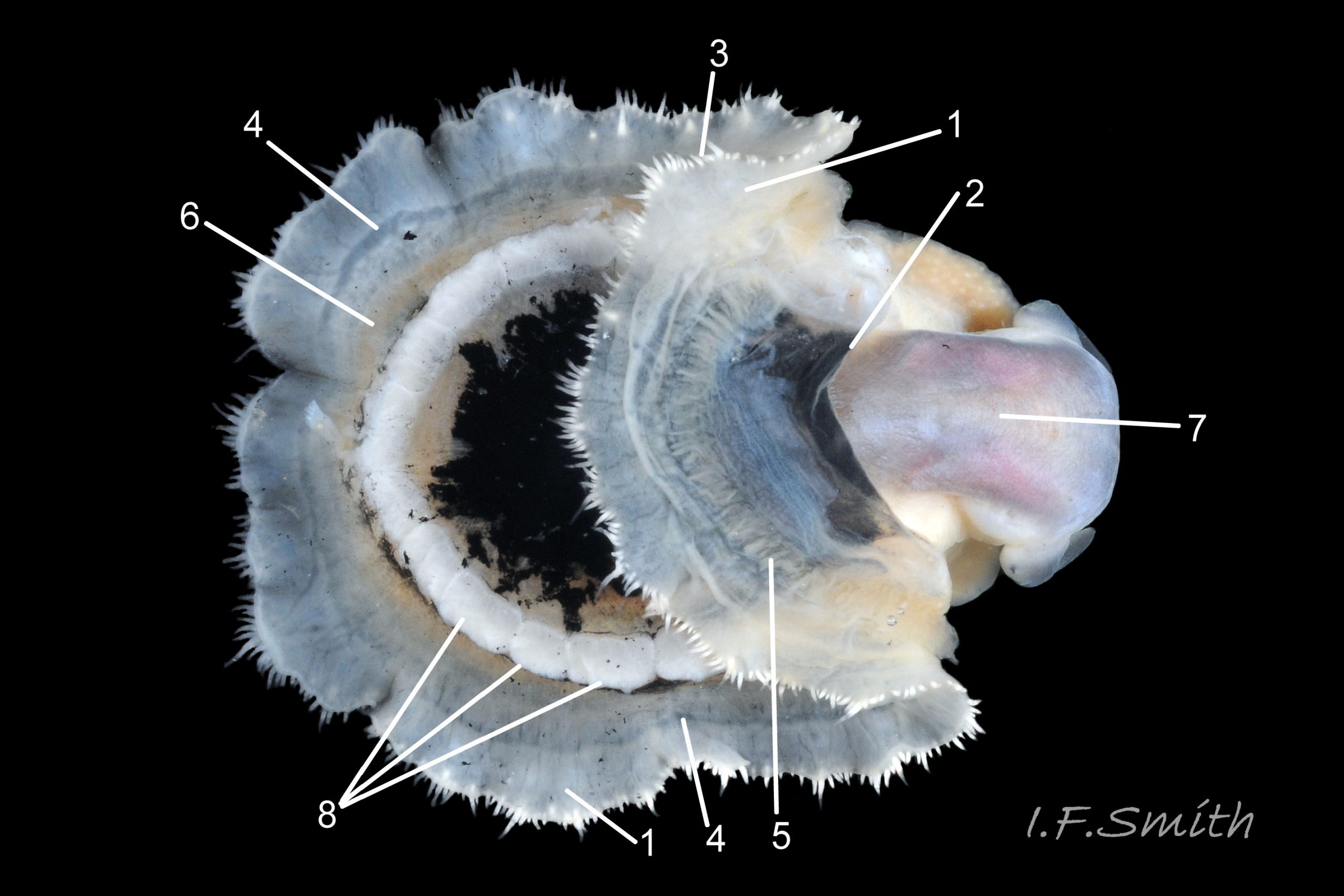
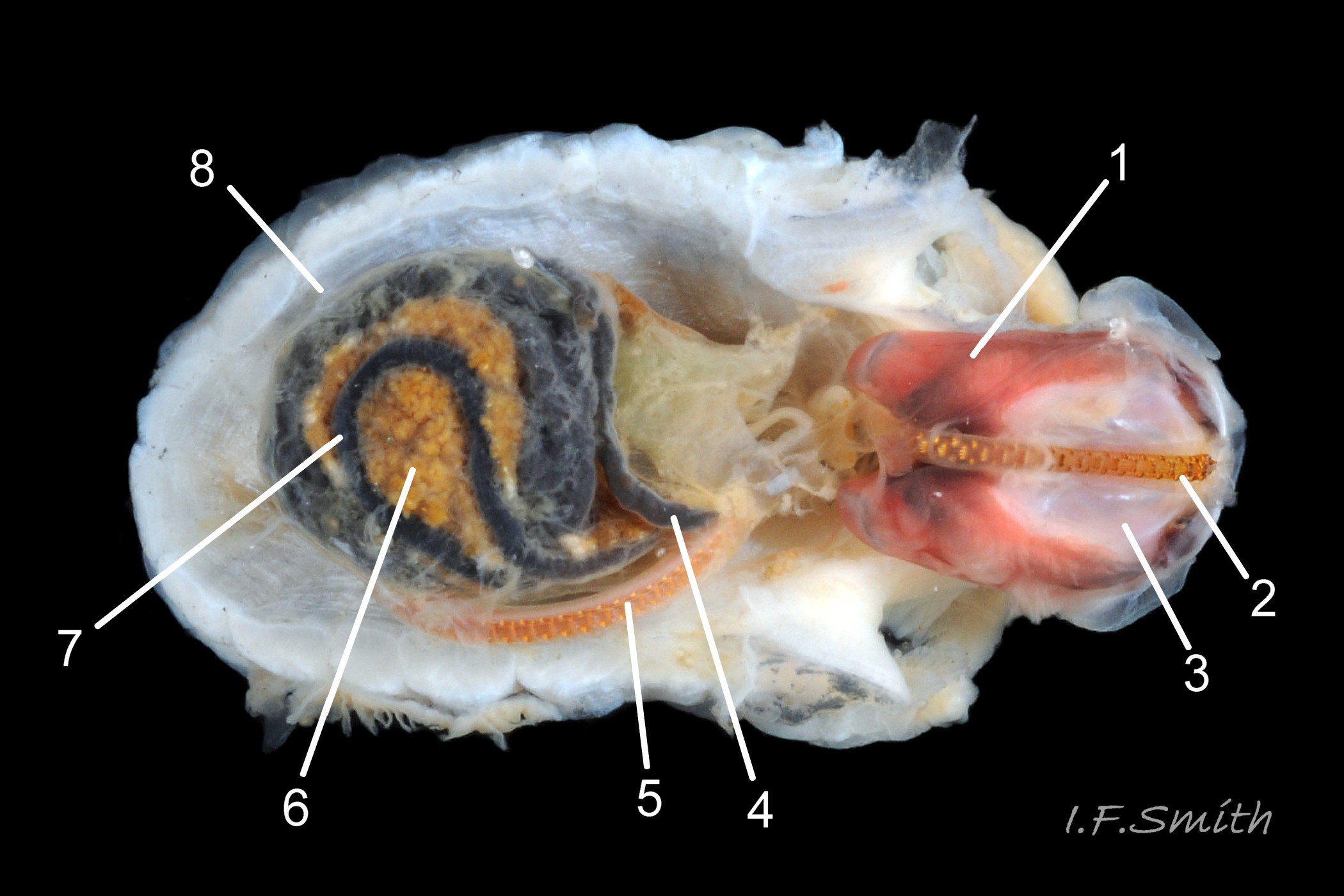
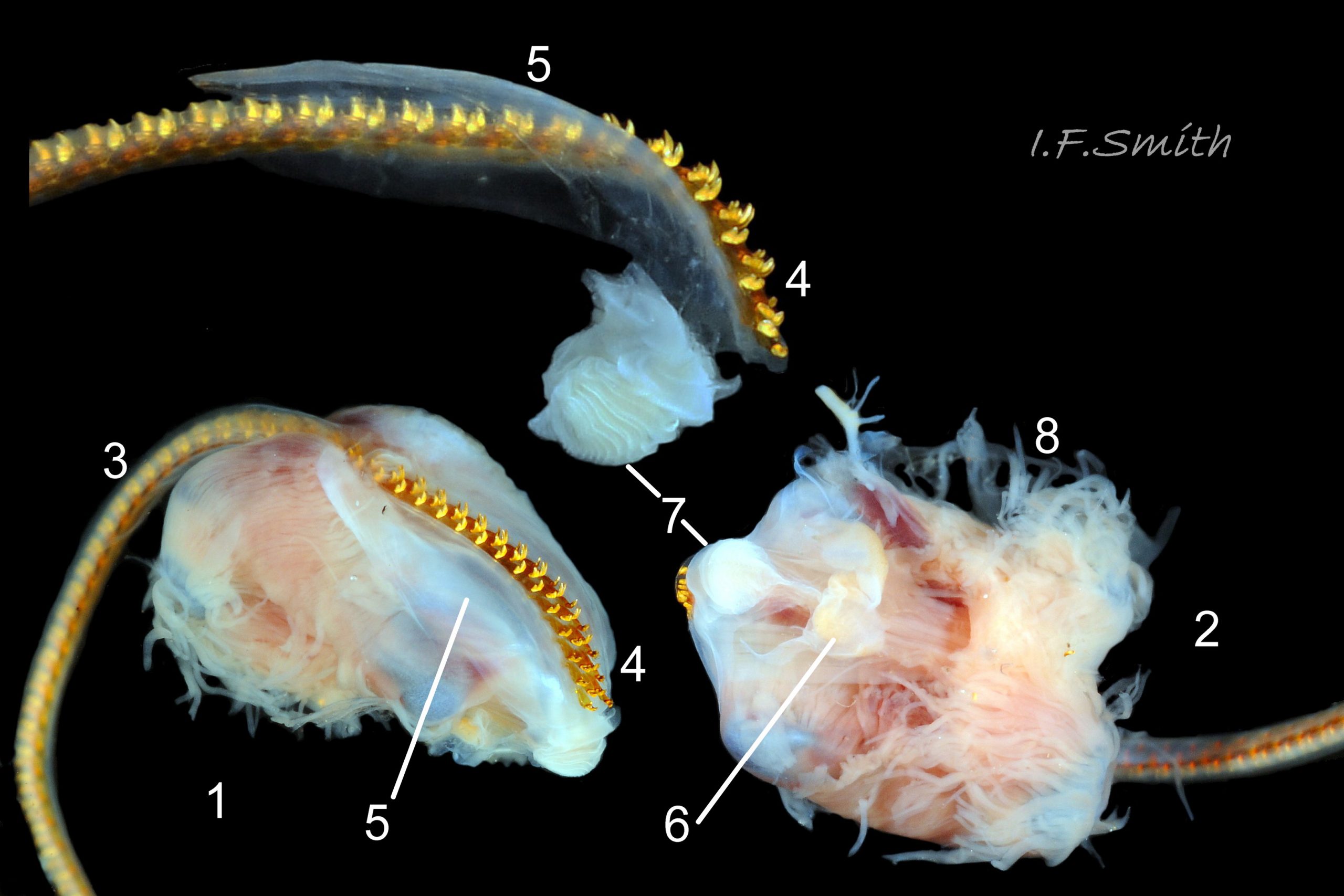
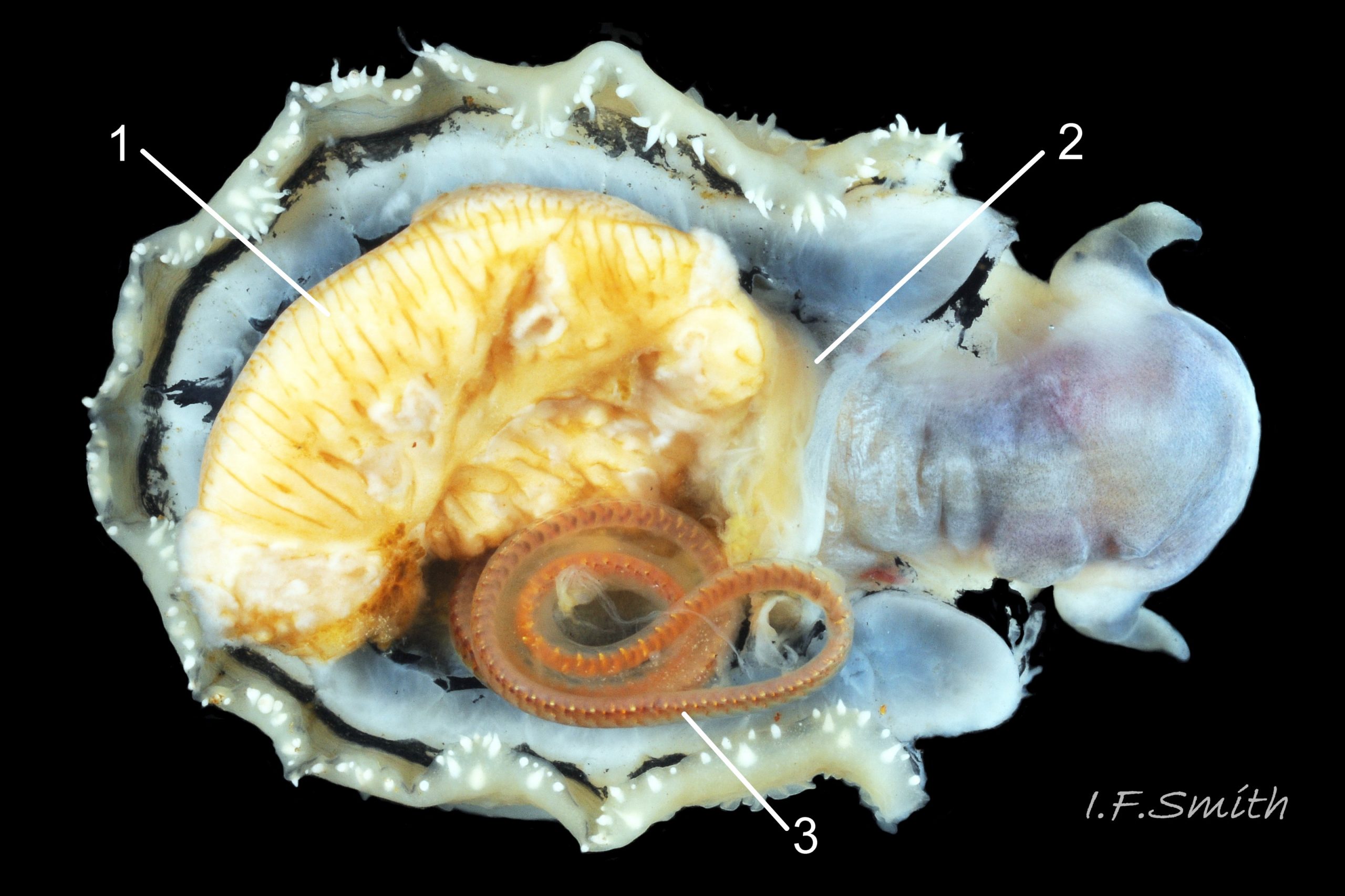
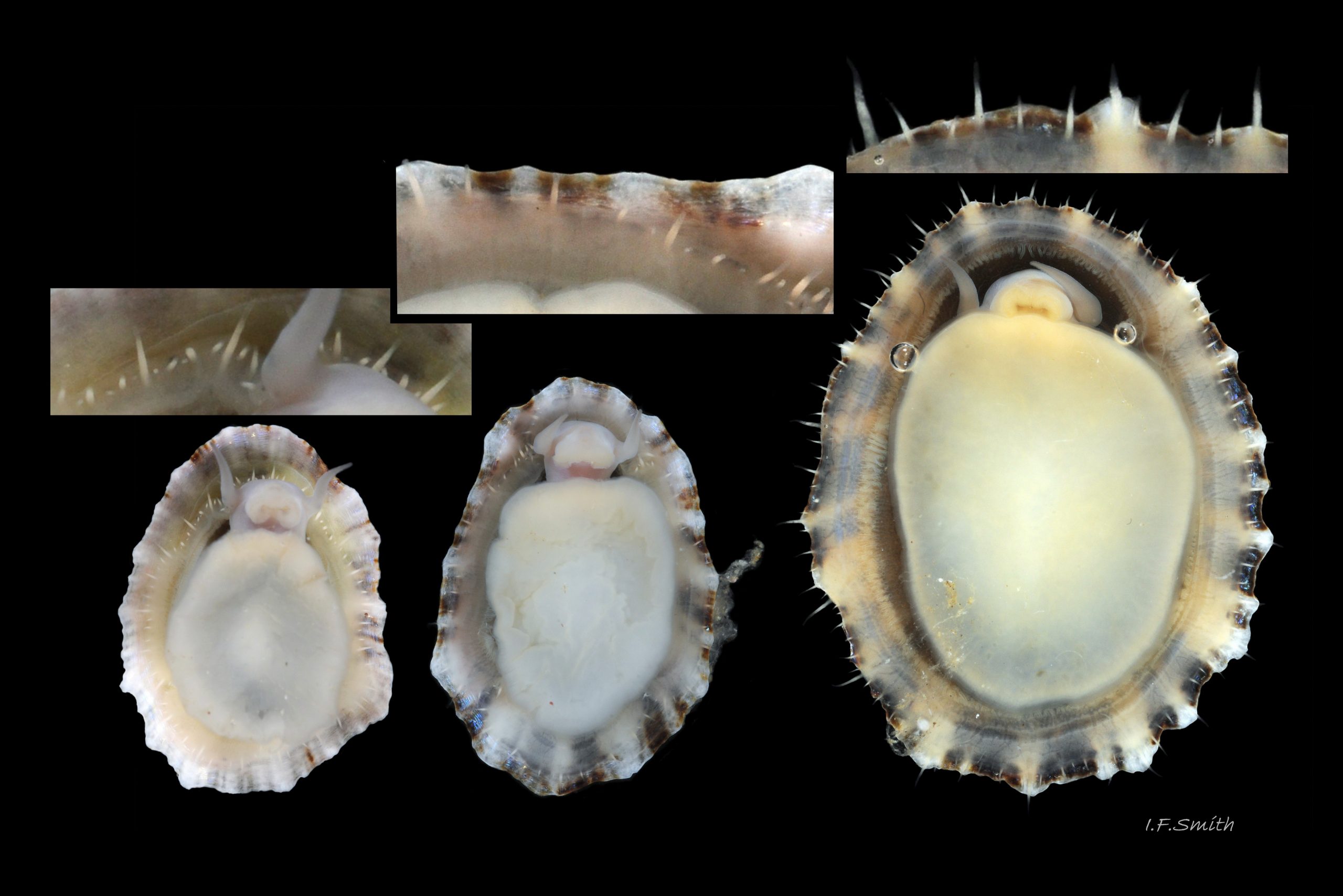
Translucent white head, darkened to purple-pink by internal odontophore 14 Patella depressa; has substantial snout , slit at posterior, with large mouth (transverse when shut) fringed by thick outer lips 15 Patella depressa. Distal end of snout and lips tinted pale yellow 14 Patella depressa. When outer lips opened, dull-yellow inner lips exposed. Inner lips open laterally to expose radula with crimson ribbon and golden teeth, and white, cuticularized, triangular licker 15 Patella depressa. Sturdy pale grey cephalic tentacles have small black eye in slight swelling at base 02 Patella depressa. Eye is primitive (or degenerate) cavity, open to seawater and lined with black retina cells. Mantle-skirt translucent buff 16 Patella depressa; colour most saturated and translucency least when skirt retracted from shell-periphery 17 Patella depressa. Mantle cavity consists of nuchal cavity over head, and pallial groove filled with pallial gills around entire periphery of foot-head 16 Patella depressa; no ctenidium. Each gill is tongue-shaped leaflet attached by stalk to distal wall of pallial-groove, and has densely ciliated groove on stalk and thickened rim 18 Patella depressa . Efferent pallial vessel in mantle-skirt, close to pallial-groove, enters nuchal cavity on left 16 Patella depressa. Mantle-edge has many opaquely-pigmented chalky-white pallial tentacles, becoming translucent and less intensely coloured only in a small distal portion; tentacles distinct from translucent, buff mantle-skirt that they arise from 19 Patella depressa. Around perimeter, pallial tentacles alternate single long with several short. Length of pallial tentacles, and their position relative to shell, vary with degree of extension of mantle skirt 20 Patella depressa, 21 Patella depressa and 17 Patella depressa. Pedal-retractor muscle arranged in horseshoe pattern of white muscle bundles demarcated by gaps 20 Patella depressa; muscle attaches body/foot to shell (a.k.a. shell muscle) 17 Patella depressa. Sole of foot approximately circular 20 Patella depressa to oval with slightly tapered posterior 21 Patella depressa , pitch-brown to black with pale peripheral rim, colour most saturated when foot contracted 17 Patella depressa White sides of foot lack features such as epipodial tentacles 02 Patella depressa. When crawling, usually only extended pallial tentacles and, perhaps, tips of cephalic tentacles protrude beyond shelter of shell 22 Patella depressa. No penis as fertilization external.
Identification of British patellid limpets.
Shell-exterior cannot be relied on, and shell-interiors can be confusing. Examination, in good light under magnification, of extended pallial tentacles on living animals is essential for consistent accurate discrimination of the three rock-dwelling Patella species. Best achieved with specimen adhering to underside of supported glass-sheet in black-based container of seawater.
Some morphologically intermediate forms can only be reliably identified by sequencing DNA or allozyme study 36 Patella depressa and 37 Patella depressa. Intermediates result from similar environmental factors affecting different species in similar ways and are not hybrids (Sanna et al., 2011 and Sá-Pinto et al., 2007). For the purpose of recording for distribution schemes it is advisable to disregard intermediates unless DNA or allozymes can be employed, especially beyond or on the limits of known distributions. Intermediates most frequent near limit of distributions of P. depressa and P. ulyssiponensis in Isle of Wight , perhaps because conditions not optimal (Fretter and Graham, 1994).
Key identification features of typical British specimens.
Patella depressa
[1&2 in combination, not singly, diagnostic of typical specimens but excludes intermediates.]
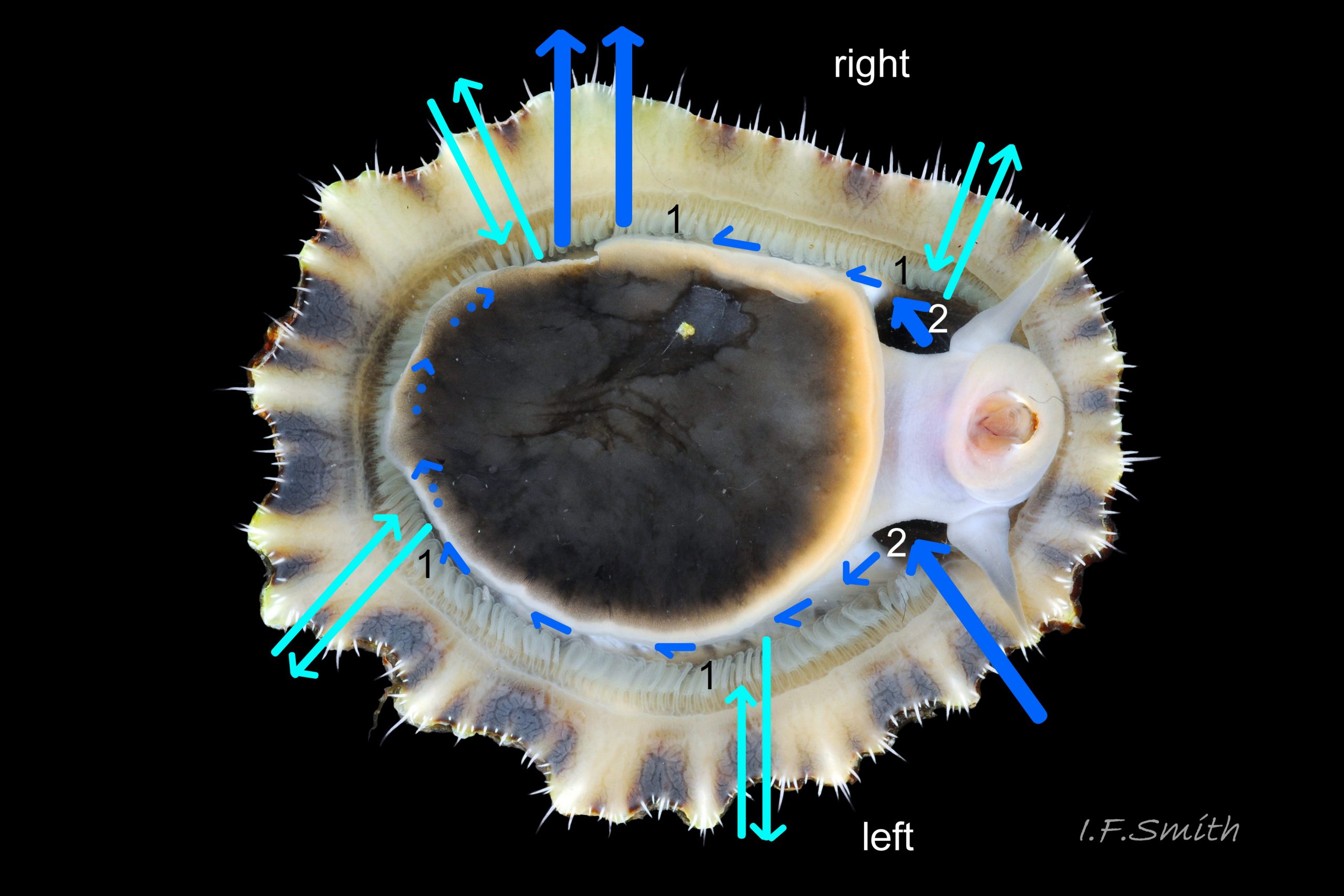
1. Pigmented pallial tentacles are opaque chalky-white for more than half of extended-length; may have translucent tip; distinctly whiter than buff mantle-skirt from which they arise 19 Patella depressa. Even when mantle-skirt retracted, pallial tentacles often clearly visible contrasting with the darker mantle 17 Patella depressa.
2. Sole of foot pitch-brown 16 Patella depressa to black 17 Patella depressa. .
3. On shell-interior, whitish projecting points of ribs have short, unglazed, chalky, pure-white central line, but reduced or lacking where projecting points of ribs eroded 08 Patella depressa. [This feature recently recognised by S. Payne, and applies to all in large sample examined by IFS. ]
Before making records of this species further north than NW Anglesey it is advisable to familiarize oneself with specimens in areas where it is known to be frequent such as S. Devon, Channel Islands or Brittany.
Confirmation/correction can be sought by posting clear photo of pallial tentacles and foot on British Marine Molluscs Group at www.facebook.com/groups/british.marine.mollusca/
Similar species
Patella ulyssiponensis
1. Pallial tentacles have opaque pigment; white, off-white, cream or, on large specimens, yellowish or orange for about half of length; distal-half fades to translucent. Opaque basal parts often distinct from translucent mantle-skirt that they arise from so possible to confuse with P. depressa; important to use pallial tentacles in combination with foot-colour/shell-length for identification 41 Patella depressa.
2. Foot not pitch-brown/black; whitish when young 42 Patella depressa becoming yellowish 43 Patella depressa and often orange with age 44 Patella depressa. Beware of juveniles under 12mm length that lack gonads above sole as dark viscera may show as blackish-shadow through thin pale translucent foot 42 Patella depressa. Green ovaries resting on interior surface of foot of mature female may show as faint greenish tinted zone along median line of foot where it is thinnest 43 Patella depressa .
Patella vulgata
Extremely variable species; foot colours and nearly all shell-features have overlaps with P. depressa and P. ulyssiponensis.
1. Pigment-less pallial tentacles are slender, translucent and same colour as mantle-skirt they arise from 45 Patella depressa.
Cautions:
Pallial tentacles may look white when arise from colourless mantle-skirt in some lighting, but no pigment and not chalky-white 46 Patella depressa .
Pigment-less translucency and fineness often make discernment difficult, especially when mantle skirt retracted from shell-rim and pallial tentacles viewed against shell 47 Patella depressa; often virtually invisible when out of water as may be retracted into mantle 48 Patella depressa.
Foot colour varies, sometimes as dark as on P. depressa 47 Patella depressa
Habits and ecology
On rocky shores with mild winters (January mean air temperature 42°F/5.6°C and above 40 Patella depressa) with Ballantine (1961) wave exposure scale 1-5 (extremely exposed to fairly sheltered) where turbidity does not prevent plant growth. On bedrock in shallow pools, seawater trickles or other damp positions, not on shingle or boulders. Not sublittoral; lower limit varies, usually somewhere between MLWN and MLWS; upper limit MHWN, or EHWS if frequent swell-splash or in pools. Average size of adults decreases up shore (Branch, 1981a); unable to produce very large, thick, high-domed shells, like those of some P. vulgata 49 Patella depressa, to resist dessication on drained rock on upper shore, though some intermediate forms show a tendency towards a high profile 36 Patella depressa. P.depressa is reported to be a rigidly homing species (Branch, 1981a), adults always after feeding-excursions seeking to return to same position. Foot longitudinally divided on interior by deep median groove of large blood sinus 25 Patella depressa. Locomotion by retrograde waves alternating on each side (ditaxic) of sole; muscles alternately compress/relax against blood trapped between them to create waves 25 Patella depressa. Feeds, mainly at night (Branch, 1981a) , on algal sporelings, detritus containing diatoms and organic remains, and on short algal growths, including encrusting calcareous spp. Grazing rock surfaces is facilitated by powerful muscles on large buccal mass 32 Patella depressa, and by hard, iron-mineralized teeth on long ribbon with plentiful replacements for worn teeth 33 Patella depressa. Length varies seasonally; shorter when wear of active feeding exceeds growth rate. Patella spp. wear out up to two rows of teeth per day (Sigel, 2008 ). About four rows of teeth are in contact with substrate during feeding 15 Patella depressa; loose particles are retained by rim of surrounding jaw# 24 Patella depressa and the licker 32 Patella depressa which sweeps them up at the end of the radula stroke. Long coiled intestine 30 Patella depressa compacts faeces into firm faecal strings that will not contaminate gills in pallial groove; compensates for adults lacking hypobranchial gland to produce mucus to bind particles exiting from nuchal cavity. Cilia on roof of nuchal cavity and side of foot conduct faecal matter from anus in nuchal cavity to middle of right side 38 Patella depressa. Faeces and debris accumulate there until periodic sharp contraction of pedal-retractor muscle clamps shell down and forcefully flushes water and waste out of shell (Fretter & Graham 1962 & 1994). When limpets removed from rock, accumulated pile of faecal strings often found in position. Cilia also create inhalent water-current from left of head through nuchal cavity, where urogenital openings located, and thence carry excreta and ova/sperm to exterior. Predators reported to be able to dislodge P. vulgata shells probably take P. depressa too; they include gulls (Larus spp.), oystercatchers (Haematopus ostralegus), crabs, starfish and rats. Nucella lapillus bores through the shell, usually to the pedal-retractor muscle where the adjacent viscera are accessible obliquely to its radula without having to bore through the thick amphora# shell-layers covering the viscera. Boring takes several days, but is rewarded with a large food supply, providing the Nucella isn’t dislodged before completion 39 Patella depressa . Absence or scarcity of P.depressa from very sheltered sites, where crabs are abundant, and from the sublittoral fringe, where starfish occur, might be because of greater susceptibilty of this small species to them than of the larger P. vulgata and P. ulyssiponensis. Respiration: gill-cilia create gentle local inhalent respiratory water currents all around perimeter of animal from adjacent shell-rim onto gills, and exhalent currents below gills back to shell-rim 38 Patella depressa (Yonge & Thompson, 1976). Densely ciliated groove on stalk and rim of each gill-lamella catches and removes large particles of detritus that would clog gill (Fretter & Graham, 1994) 18 Patella depressa. Blood passes from viscera and foot via vessels through gaps in encircling shell-muscle 20 Patella depressa into gills for oxygenation, and thence into encircling efferent pallial vessel 26 Patella depressa which takes it through nuchal cavity 29 Patella depressa to heart for recirculation to head, foot and viscera (Fretter & Graham, 1994) .
Breeds spring to autumn, perhaps with summer pause (Fretter & Graham, 1994). May vary geographically; Yonge & Thompson (1976) said breeds sporadically all year with summer maximum. External fertilization so close proximity of sexes required for success; new populations unlikely to be established by isolated strays. Sperm and ova shed into water column, ova individually. Eggs hatch as free trochophore larvae (stage passed within egg by most “less-primitive” spp.) in plankton before transforming to veligers and, after a short planktonic-life, settling on lower shore and assuming limpet form. Spat, when 1mm long, have ten radiating ridges; P. vulgata has five ridges on right, four on left. P. ulyssiponensis has eight (Fretter & Graham, 1994). Move higher up shore when shell-length 5mm.
Distribution and status
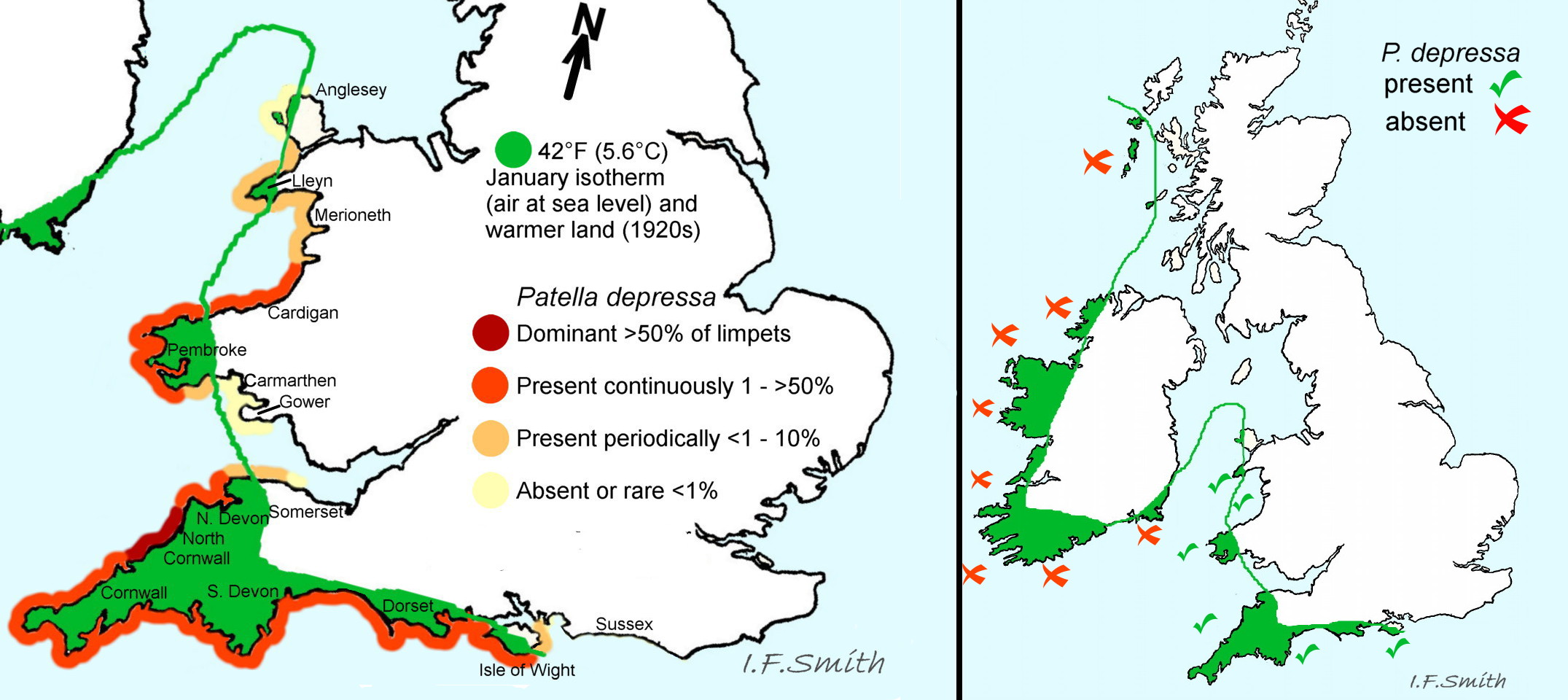
P. depressa is a North-east Atlantic species extending northwards from Senegal (Trigo et al., 2018) through western Iberia and France to its northern limits in North Wales, eastern part of south coast England and Normandy. It extends only a short way into the Mediterranean. Trigo et al. (2018) states to Malaga, but an image with diagnostic foot and pallial tentacles shows it extends to Almeira 51 Patella depressa Its absence further into the Mediterranean might be because the slight tidal rise and fall there does not produce a sufficient intertidal zone for this purely eulittoral species. The precise northern limits fluctuate, generally polewards in warm periods and receding in cooler periods, though other factors may have an influence 40 Patella depressa. In Britain, it is restricted to areas with mild winters with February mean Sea Surface Temperatures above 8°C. Consistently present populations occur between the Isle of Wight and mid Wales with zones of fluctuation beyond those points (Kendall et al. 2004). The most recent published information (Oróstica et al., October 2020 ) shows that, as would be expected in this period of exceptional temperature increase, the range has extended with scattered rare specimens reaching Beachy Head in south-east England, near Dunster in Somerset and Barry in Glamorgan but, unexpectedly, it has not reoccupied the north-western side of Lleyn or north-west Anglesey where it was recorded in the 1950s and lost in the 1980s.
The absence of P. depressa records on the mild-winter coasts of Ireland obviously requires explanation. Its distribution is used in Lewis (1964) as an exemplar of “southern species” with an explanation for its absence from Ireland. This eulittoral species cannot crawl across wide sublittoral areas. It is possible that it reached Britain in the warming after the Ice Age before sea-level rise cut Britain off from the continent but after Ireland had been cut off from Britain. Planktonic larvae can traverse sublittoral areas, but the phase is short in P. depressa so time is limited and it is difficult if currents are adverse. External fertilization requires a minimum population density with the sexes in close proximity for successful establishment of a sustained population; there may have been occasional small settlements in Ireland that have subsequently failed (Lewis, 1964). Records in Ireland, Scotland and the North Sea on distribution maps should be regarded as misidentifications unless there is photographic evidence of both brown-black foot and chalk-white opaque pallial tentacles.
Acknowledgements
I gratefully acknowledge the help of Jan Light and Sebastian Payne with information and discussion, and in providing many specimens for examination. Any errors or omissions are the responsibility of the author. I thank Samuel Santos García for use of his photograph of a specimen from Almeira.
Links and references
Akşit, D. & Falakil Mutaf, B. 2011. The external morphology of the gill of Patella caerulea L. (Mollusca: Gastropoda). Turk. J. Zool. 35(4) 603-606. Tübitak. Turkey. PDF contains SEM images of gill lamellae.
www.google.co.uk/search?q=patella+gill+ciliated+groove&am…
Ballantine, W.J. 1961. A biologically-defined exposure scale for comparative description of rocky shores. Field studies 1(3): 1-19. Free pdf at: fsj.field-studies-council.org/media/344345/vol1.3_17.pdf
Barber, A.H., Lu, D. & Pugno, N.M. 2015 Extreme strength observed in limpet teeth The Royal Society.
rsif.royalsocietypublishing.org/content/12/105/20141326
Branch, G.M. 1981a. The biology of limpets. Oceangr. Mar. Biol. Ann. Rev. learning.watfordboys.org/mod/resource/view.php?id=4730
Branch, G.M. 1981b. The biology of limpets. Oceangr. Mar. Biol. Ann. Rev. www.google.co.uk/search?q=Patella+vulgata+blood+circulati...
Cohen, A.L. & Branch, G.M. 1992. Environmentally controlled variation in the structure and mineralogy of Patella granularis shells from the coast of southern Africa: implications for palaeotemperature assessments. Palaeogeography, palaeoclimatology, palaeoecology, 91: 49-57. www.whoi.edu/fileserver.do?id=163844&pt=2&p=36767
Forbes, E. & Hanley S. 1849-53. A history of the British mollusca and their shells. vol. 2 (1849), London, van Voorst. (As dark-bodied variety of their P. athletica) ; Free PDF at archive.org/stream/historyofbritish02forb#page/428/mode/2up Use slide at base of page to select pp.428 for footnote.
Fretter, V. and Graham, A. 1962. British prosobranch molluscs. London, Ray Society.
Fretter, V. and Graham, A. 1994. British prosobranch molluscs. Revised and updated edition. London, Ray Society.
Goshima, S., Ilano A.S. & Ito, A. 2002. Seasonal and tidal-height variations in body weight and radular length in Nodilittorina radiata (Eydoux & Souleyet, 1852) J. Mollus. Stud. 68(3): 197-203.
mollus.oxfordjournals.org/content/68/3/197.full.pdf+html
Graham, A. 1988. Prosobranch and pyramidellid gastropods. London.
Heppel, D. 1964. Recorder’s report: marine Molluscs. J. Conch. Lond., 25: 308-313.
Hughes, S.L., Holliday, N.P., Kennedy, J., Berry, D.I., Kent, E.C., Sherwin, T., Dye, S., Inall, M., Shammon, T. and Smyth, T. 2010. Temperature (Air and Sea) in MCCIP Annual Report Card 2010-11, MCCIP Science Review, 16pp. www.mccip.org.uk/arc
Jeffreys, J.G. 1862-69. British conchology. vol. 3 (1865). London, van Voorst. (As var.3 intermedia of Patella vulgata. Note that var.4 depressa describes P. ulyssiponensis features); Free PDF at archive.org/stream/britishconcholog03jeff#page/230/mode/2up . Use slide at base of page to select pp.230- 241.
Lewis, J.R. 1964. The ecology of rocky shores. London, Hodder & Stoughton.
Light, J. A guide to limpet identification for the general naturalist www.glaucus.org.uk/Limpet.htm
MacClintock, C. 1967. Shell structure of patelloid and bellerophontid gastropods (Mollusca). Peabody Museum of Natural History, Yale University. Bulletin 22.pdf at
www.google.co.uk/?gws_rd=ssl#q=MacClintock%2C+C.+1967.+Sh….
215 pages, may take a few minutes to download. Contents on page v.(= p.6 of pdf). To find pages on pdf add 1 to Roman numerals, and add 11 to Arabic numerals.
McKay, D.W. and Smith, S.M. 1979. Marine mollusca of East Scotland. Edinburgh, Royal Scottish Museum.
Moore, H.B. 1937. Marine fauna of the Isle of Man. University Press of Liverpool. (“P. depressa Pennant” listed for I.O.M., but “[= P. athletica Bean] Forbes & Hanley 1853” shows record is actually of P. ulyssiponensis following the taxonomic misunderstanding of Jeffreys, and Forbes & Hanley).
Oróstica, M. H., Hawkins, S. J., Broitman, B. R. and Jenkins S. R. 2020. Performance of a warm-water limpet species towards its poleward range edge compared to a colder-water congener. Mar Ecol Prog Ser doi.org/10.3354/meps13461
Pennant, T. 1777 British zoology London.
Page 142 biodiversitylibrary.org/item/127011#page/168/mode/1up
Pl. 89 46. biodiversitylibrary.org/item/127011#page/361/mode/1up
Sanna, D., Dedola, G. L., Lai, T., Curini-Galletti, M. & Casu, M. 2011. PCR-RFLP: A practical method for the identification of specimens of Patella ulyssiponensis s.l. (Gastropoda: Patellidae), Italian Journal of Zoology,
pdf at www.researchgate.net/publication/233126771_PCR-RFLP_A_pra…
Sá-Pinto, A., Branco,M., Harris, D.J. & Alexandrino, P. 2005. Phylogeny and phylogeography of the genus Patella based on mitochondrial DNA sequence data. J. Exp. Mar. Biol. Ecol. 325: 95-110.
Sá-Pinto, A., Alexandrino, P. & Branco,M. 2007. High genetic differentiation with no evidence of hybridization between four limpet species (Patella spp.) revealed by allozyme loci. Scientia Marina 71(4): 801-810. Barcelona. pdf at www.vliz.be/imisdocs/publications/131981.pdf
Sigel, A., Sigel, H. and Sigel, R.K.O., (Editors) 2008. Biomineralization: from nature to application. Chichester, Wiley. Page 299 (to access this page on-line Google-search “limpet wear teeth” and scroll down results to find title) books.google.co.uk/books?id=TiwK2VQhPMkC&pg=PA299&…
Tomlin, J.R.Le B. 1923. Patella depressa Pennant. J. Conch. Lond., 17: 34.
Trigo, J.E.; Diaz Agras, G.J.; Garcia Alvarez, O.L.; Guerra, A.; Moreira, J.; Pérez, J.; Rolán, E.; Troncoso, J.S,; Urgorri, V.. 2018. Guia de los Moluscos Marinos de Galicia. Servicio de Publicacións da Universidade de Vigo.
Yonge, C.M. and Thompson, T.E. 1976. Living marine molluscs. London.
Current taxonomy: World Register of Marine Species (WoRMS)
www.marinespecies.org/aphia.php?p=taxdetails&id=151374
GLOSSARY
amphora – (on interior of limpet shell) Roman amphora-shaped area enclosed by scars of pedal-retractor muscle and anterior mantle-attachment.
aperture – mouth of gastropod shell; outlet for head and foot.
apex – earliest formed part of a gastropod shell, the summit of the cone. (In this limpet-account restricted to the exterior of the shell, and “vertex” used for the interior.)
cephalic – (adj.) of or on the head.
cilia – (pl.) microscopic linear extensions of membrane that move in rhythmic waves to create locomotion, or move particles and liquids e.g. inhalent water currents. (“cilium” singular). (Electron scanning microscope image at 18 Lepidochitona cinerea )
ciliary – (adj.) relating to or involving cilia.
coll. – in the collection of (named person or institution) (compare with legit).
conoid – shaped like a cone.
ctenidium – comb-like molluscan gill; usually an axis with a row of filaments either side (missing from Patella spp.).
distal – away from centre of body or point of attachment.
ditaxic – (of locomotion waves on foot) double series of waves, out of phase with each other, one series on each side of median line on sole.
ELWS – extreme low water spring tide level (usually near March and September equinoxes).
EHWS – extreme high water spring tide level (usually near March and September equinoxes).
epipodial – (adj.) of the epipodium (collar or circlet running round sides of foot of some gastropods).
epithelium – membranous covering of internal and external surfaces of animal’s body, e.g. skin and lining of tubes and cavities.
head scar – term used by many British authors for patch of different shell-material, and often different colour, near vertex of interior of limpet shell; misnomer as the mobile head, free of any attachment to the shell or mantle-roof of the nuchal cavity cannot make a scar. A preferable term is “vertex patch”.
height – (of limpet) perpendicular distance from apex to plane of aperture-rim (best measured with callipers).
hyaline shield – transparent sheet of chitin at anterior of radula that rests on bolsters of odontophore; attachment point for retractor muscles of radula; helps guide food particles into mouth.
interspecific – existing or arising between different species.
intraspecific – occuring within a single species or involving members of one species.
jaw – unarticulated chitinous structure that encloses inner lips of Patella spp. at sides and anterior.
legit – (abbreviation; leg.) collected/ found by (compare with coll.)
licker – cuticularized structure with plate-like ridges and deep transverse grooves at tip of radula of Patella spp.; retains and sweeps up food particles.
mantle – sheet of tissue covering visceral mass of molluscs. Secretes shell of shelled species, and forms part or all of dorsal body surface (notum) of those without shells. (See mantle skirt.)
mantle skirt – extension on gastropods of mantle proper as a flap roofing a cavity containing gills, genital and renal openings, anus etc. On limpets, skirt and cavity extend around periphery of animal.
MHWN – mean high water neap tide level (mean level reached by weakest high tides for a few days every fortnight. i.e. those that rise the least).
MLWN – mean low water neap tide level (mean level reached by weakest low tides for a few days every fortnight. i.e. those that fall the least).
MLWS – mean low water spring tide level (mean level reached by lowest low tides for a few days every fortnight; Laminaria or Coralline zone on rocky coasts).
nuchal – (adj.) of nape of the neck.
nuchal cavity – cavity roofed by mantle skirt that contains head of limpet; part of mantle cavity (remainder consists of pallial groove on each side of body).
ovoid – egg-shaped, as a solid or in outline.
pallial groove band – shell material deposited on interior of shell by strip of black mantle roofing the pallial groove that contains the gills. On British Patella spp. p.g. band is often clouded-white.
pedal retractor muscle – strong muscle that retracts foot into shell of most gastropods, but on limpets is used to clamp shell to substrate, a.k.a. “foot muscle”.
retrograde – (of locomotion waves on foot) waves travel from anterior to posterior.
scar – mark on shell made by attachment point of muscle or other body part.
skirt shell layer – shell material deposited on interior of shell by mantle skirt. On British Patella spp. colourless when deposited, and clouded white, or transparent showing the colours of the outer layer. Crystalline structure causes short lines of blue iridescence parallel to the aperture rim on all four British species of Patella when the light is right.
trochophore – spherical or pear-shaped larva that swims with aid of girdle of cilia. Stage preceding veliger, passed within gastropod egg in most spp. but free in plankton for patellid limpets, most Trochidae and Tricolia pullus.
tricuspid – (of tooth) having three points.
unicuspid – (of tooth) having a single point.
veliger – shelled larva of marine gastropod or bivalve mollusc which moves by beating cilia of a velum (bilobed flap).
vertex – angle at highest point on interior of limpet-shell. [Synonym of “apex”, chosen (by IFS) to help avoid confusion with the highest point, apex, on the exterior. Gmelin used “vertex” when describing the interior of Patella ulyssiponensis, and in classical Latin “vertex” was used for the “pole of the heavens”; obviously only seen from below.]
vertex patch –layer of different shell-material, and often different colour, at vertex of interior of limpet shell. (See “head scar”.)