Click image to enlarge with full caption. Main text below slider.
Lepidochitona cinerea (Linnaeus, 1767)
Current taxonomy: World Register of Marine Species (WoRMS)
www.marinespecies.org/aphia.php?p=taxdetails&id=140143
Revised, 2020, PDF version at www.researchgate.net/profile/Ian_Smith19/research
Synonyms: Chiton cinereus Linnaeus, 1776; Chiton marginatus Pennant, 1777; Lepidochiton cinereus (Linnaeus, 1776) [spelling variant];
Meaning of name Lepido = scaly, chiton= Ancient Greek tunic, cinerea= grey, so “Grey scaly tunic”.
Vernacular: Grey chiton; Lleuen lwyd y graig (Welsh); Chiton gris (French); Rändel Käferschnecke; Graue Käferschnecke (German); Asgrauwe keverslak (Dutch);
GLOSSARY BELOW This account uses the standardised terminology for chitons proposed by Schwabe (2010). Some of Jones & Baxter (1987) alternatives are indicated in the glossary as a.k.a..
Shell Description
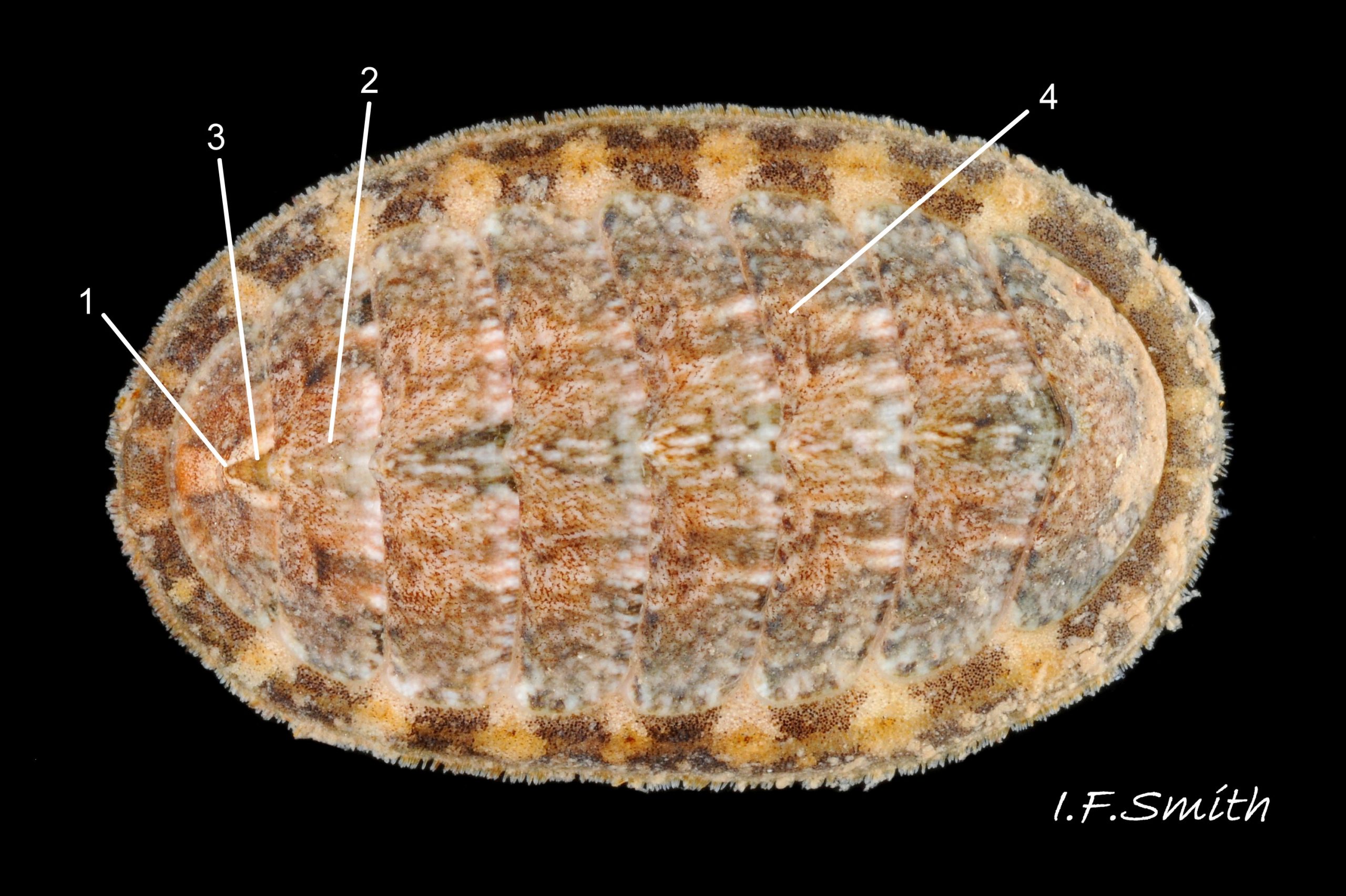
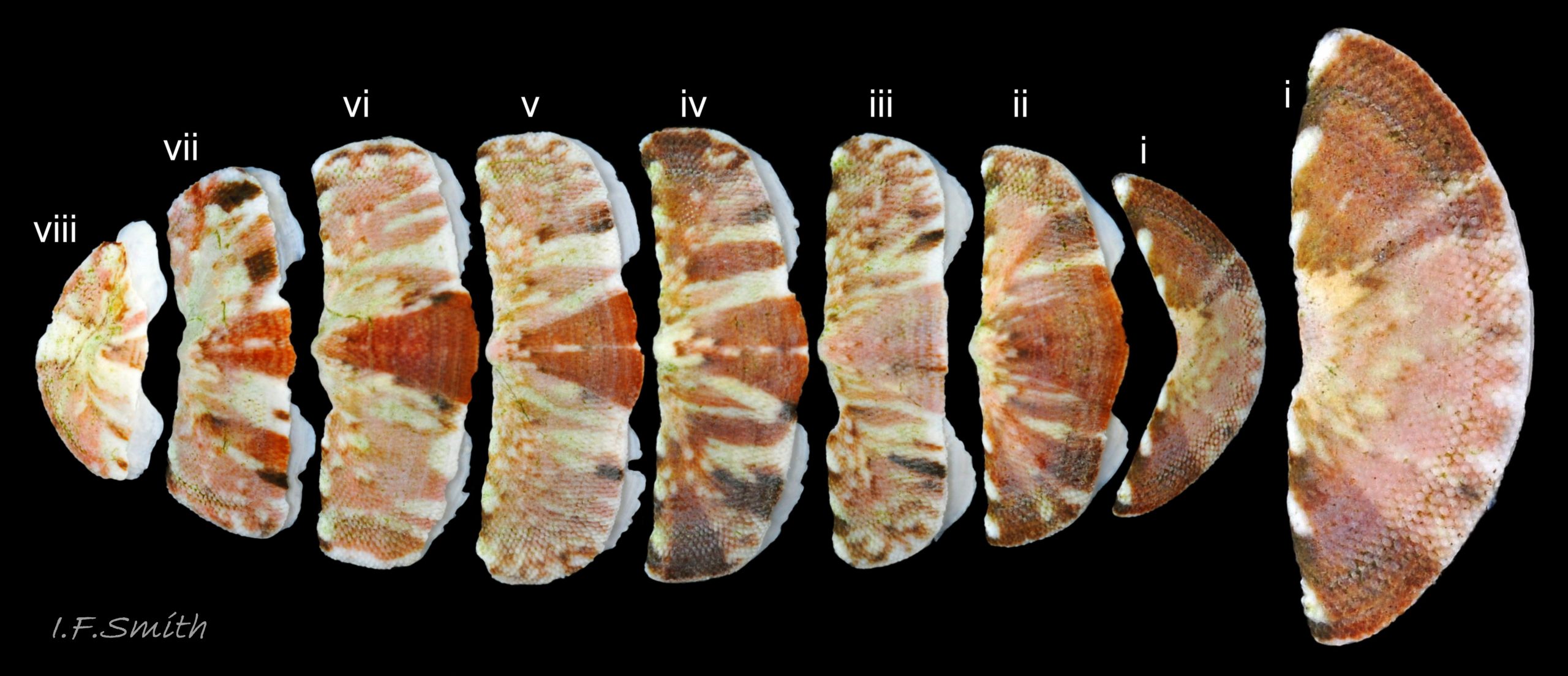
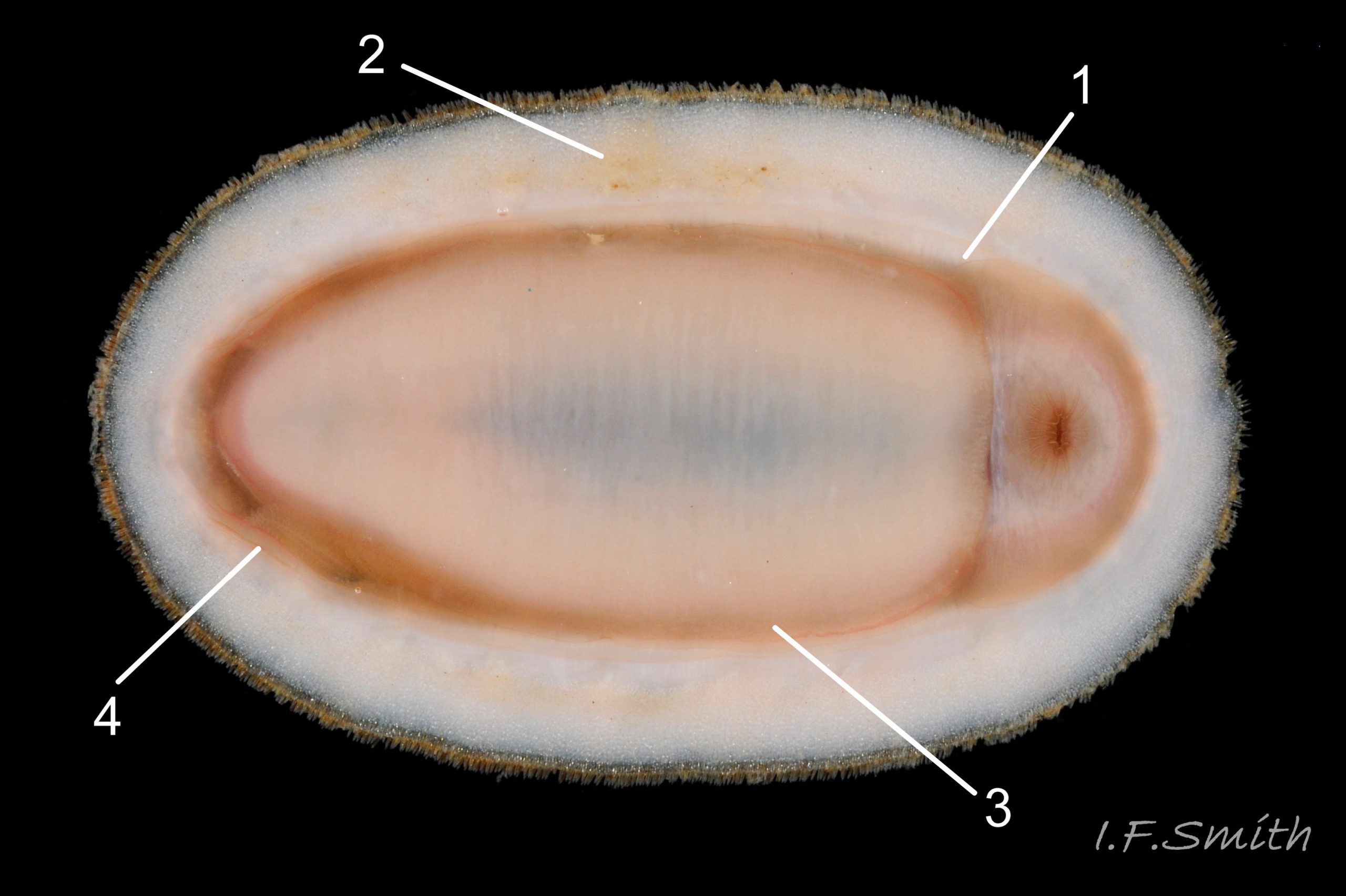
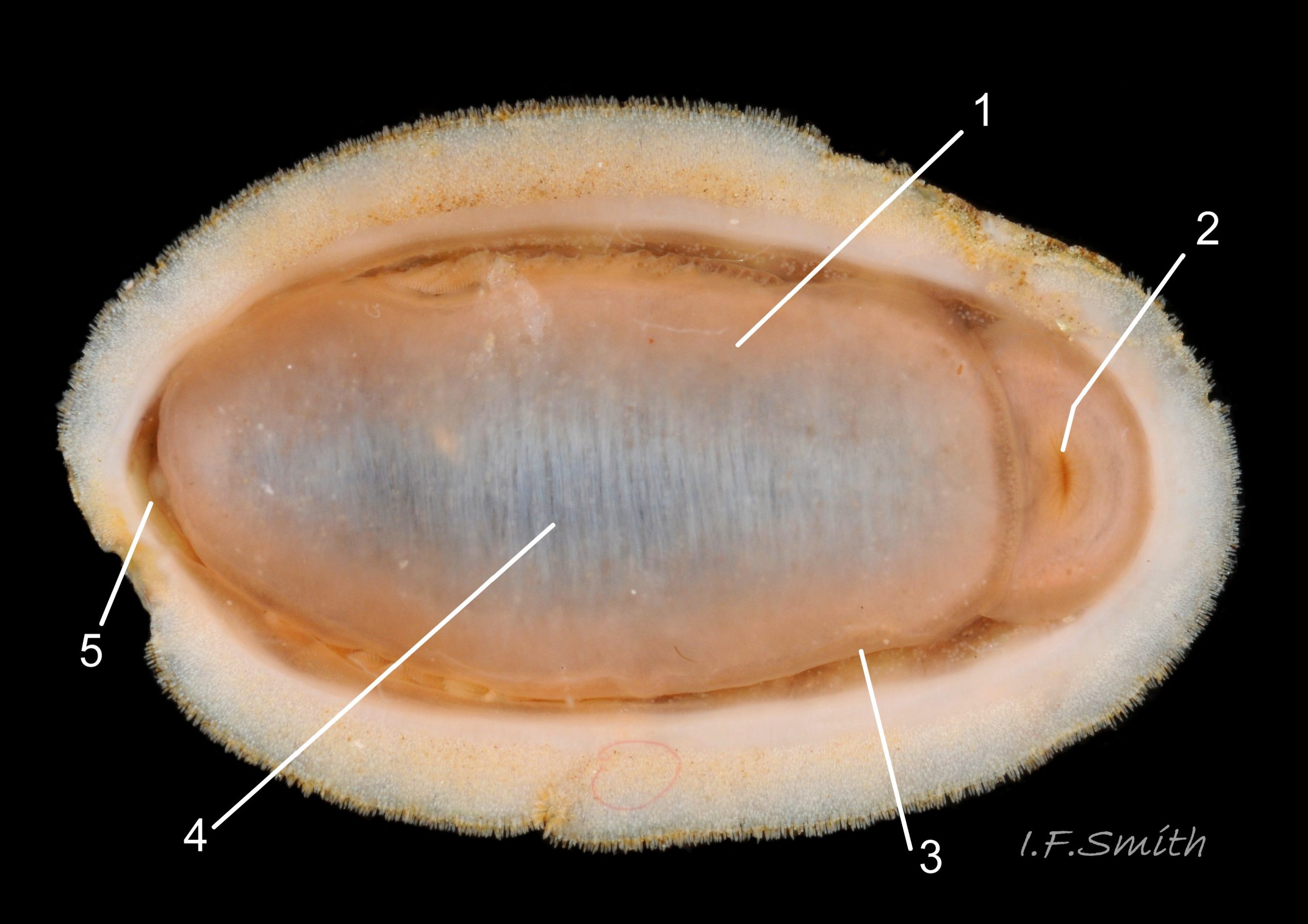
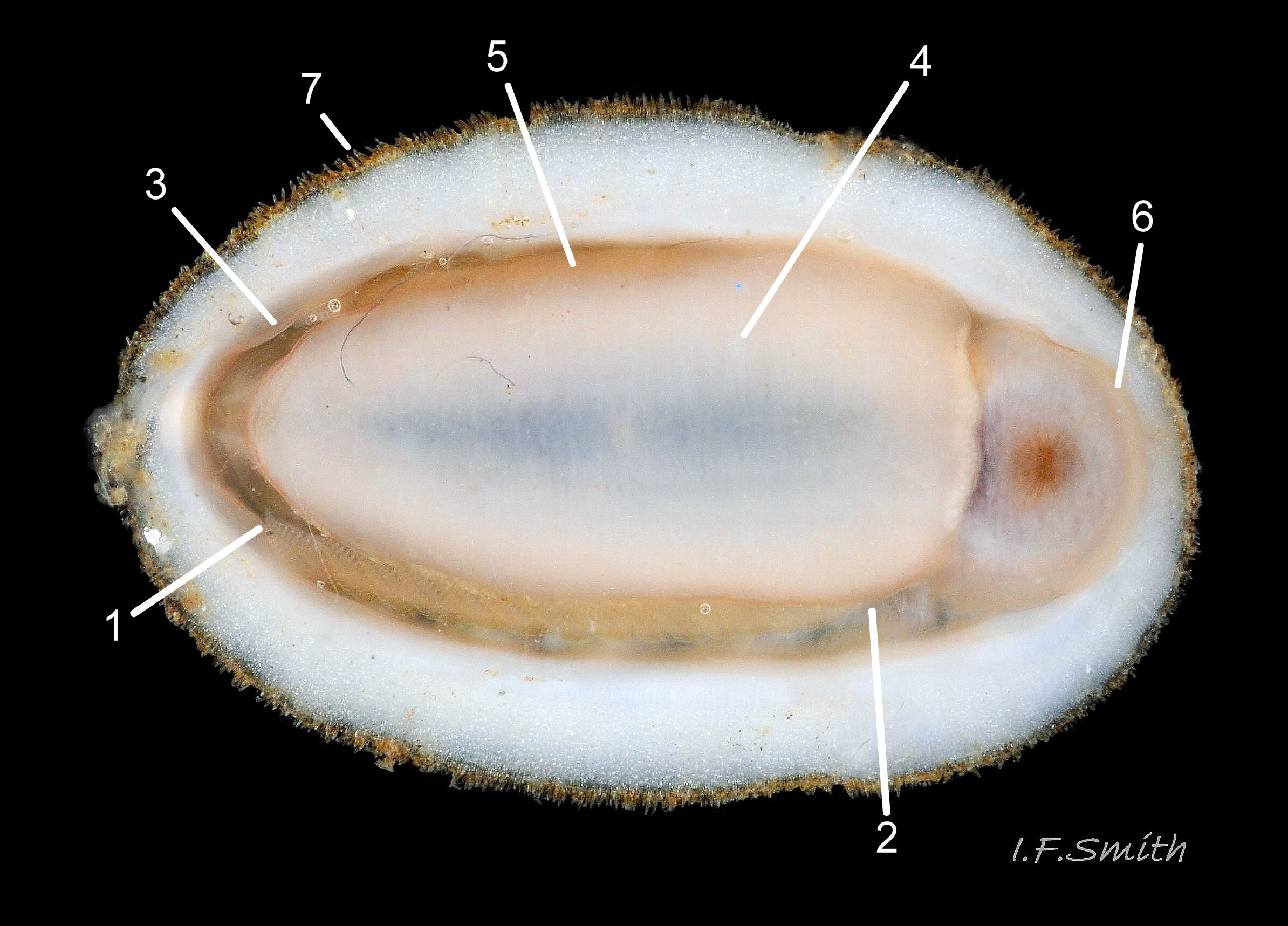
Usually 12 – 16 mm long, exceptionally to 28mm. Dorso-ventrally flattened, bilaterally symmetrical. In dorsal view, outline 01 Lepidochitona cinerea , but in lateral view, because of slope of shell and perspective, girdle appears to take up about 02 Lepidochitona cinerea. .
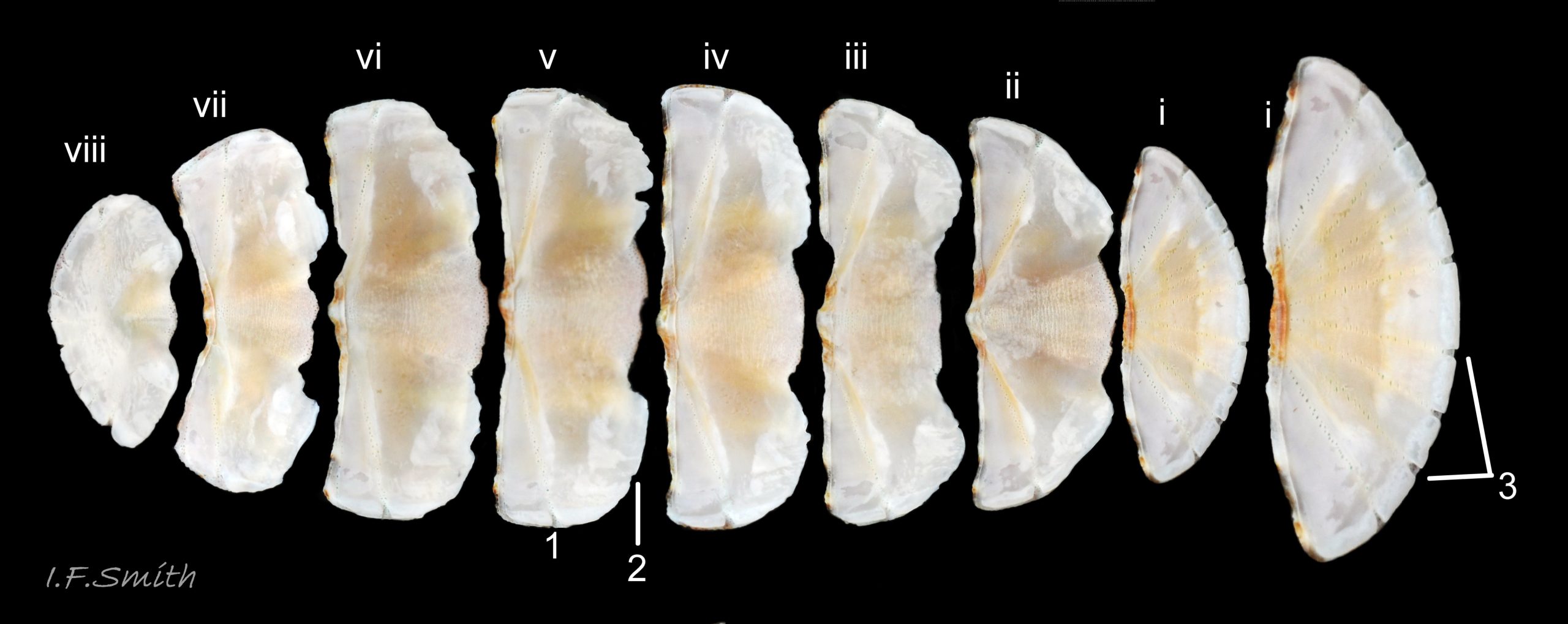
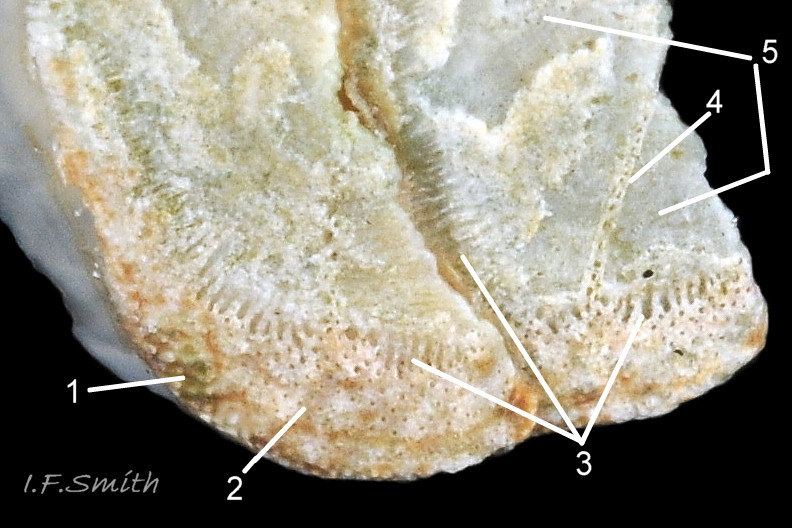
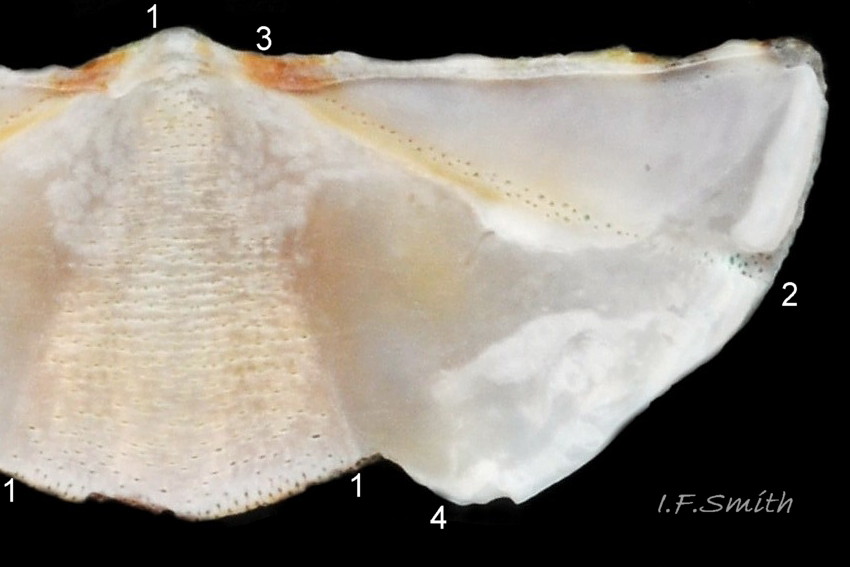
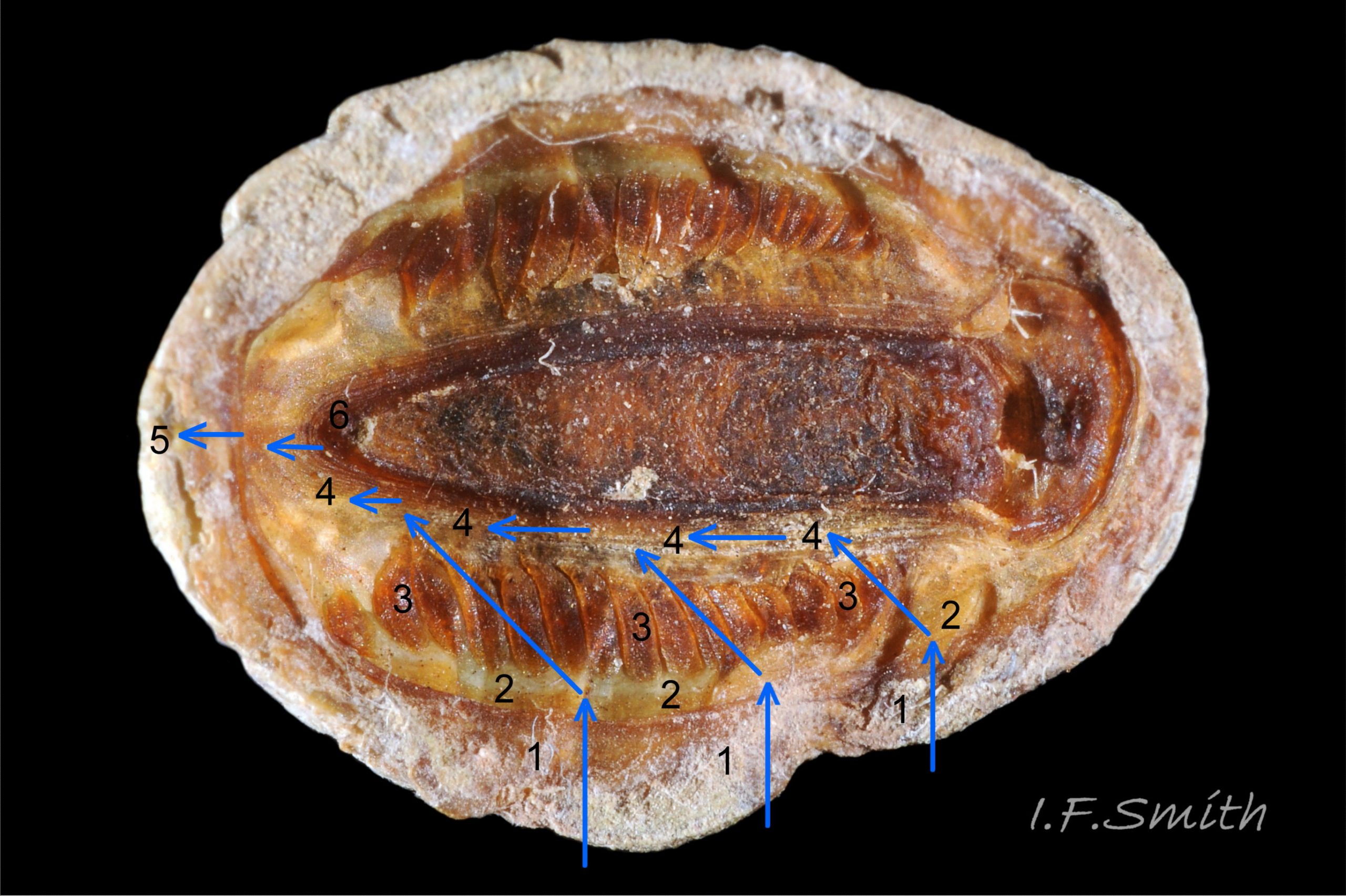
27 Lepidochitona cinerea ), principally made of 1) tegmentum: outer coloured layer of aragonite permeated and weakened 04 Lepidochitona cinerea or greenish aragonite; also 3) properiostracum: outermost proteinaceous layer containing colour and 4) myostracum: thin discontinuous innermost layer (only visible in section examined under microscope). Head valve (i) 03 Lepidochitona cinerea . Intermediate valves (ii –vii) broadly rectangular (in dorsal view, posterior edge almost straight 03 Lepidochitona cinerea ; slightly keeled (sub-angulated) with gently convex upper and middle side-slopes, and slightly 05 Lepidochitona cinerea ; noticeably higher than Tonicella 01 Lepidochitona cinerea with slightly convex, steep postmucronal slope; antemucronal area may resemble jugal area of intermediate valves 01 Lepidochitona cinerea . Slight apex/beak on each of valves ii –vii visible in lateral view, and 06 Lepidochitona cinerea and terminate on its dorsal surface in densely packed granules (visible at 5X) 09 Lepidochitona cinerea and some canals penetrate the articulamentum to form holes on its ventral surface, especially in the slit ray, and in jugal tract with about 30 undulating transverse rows of holes 07 Lepidochitona cinerea. Insertion plates of valves ii – vii embedded in girdle have a single slit; head valve (i) has about eight slits (range 7 – 11) 04 Lepidochitona cinerea , and tail valve (viii) has about eleven slits (range 6 – 16). Typical slit formula 8/1/11, but can vary. Slit rays; (rows of canal pores in ventral surface) run from slits to posterior edge of valves i – vii 07 Lepidochitona cinerea and from slits to mucro on valve viii. Valves ii – viii each have two apophyses on anterior edge 04 Lepidochitona cinerea ; those on valves iv – viii are short (i.e. antero-posterior distance), wide, near-trapezoidal, some with gently curving anterior edge. Those on valves ii and iii are more strongly curved. Apophyses extend under next valve forwards.
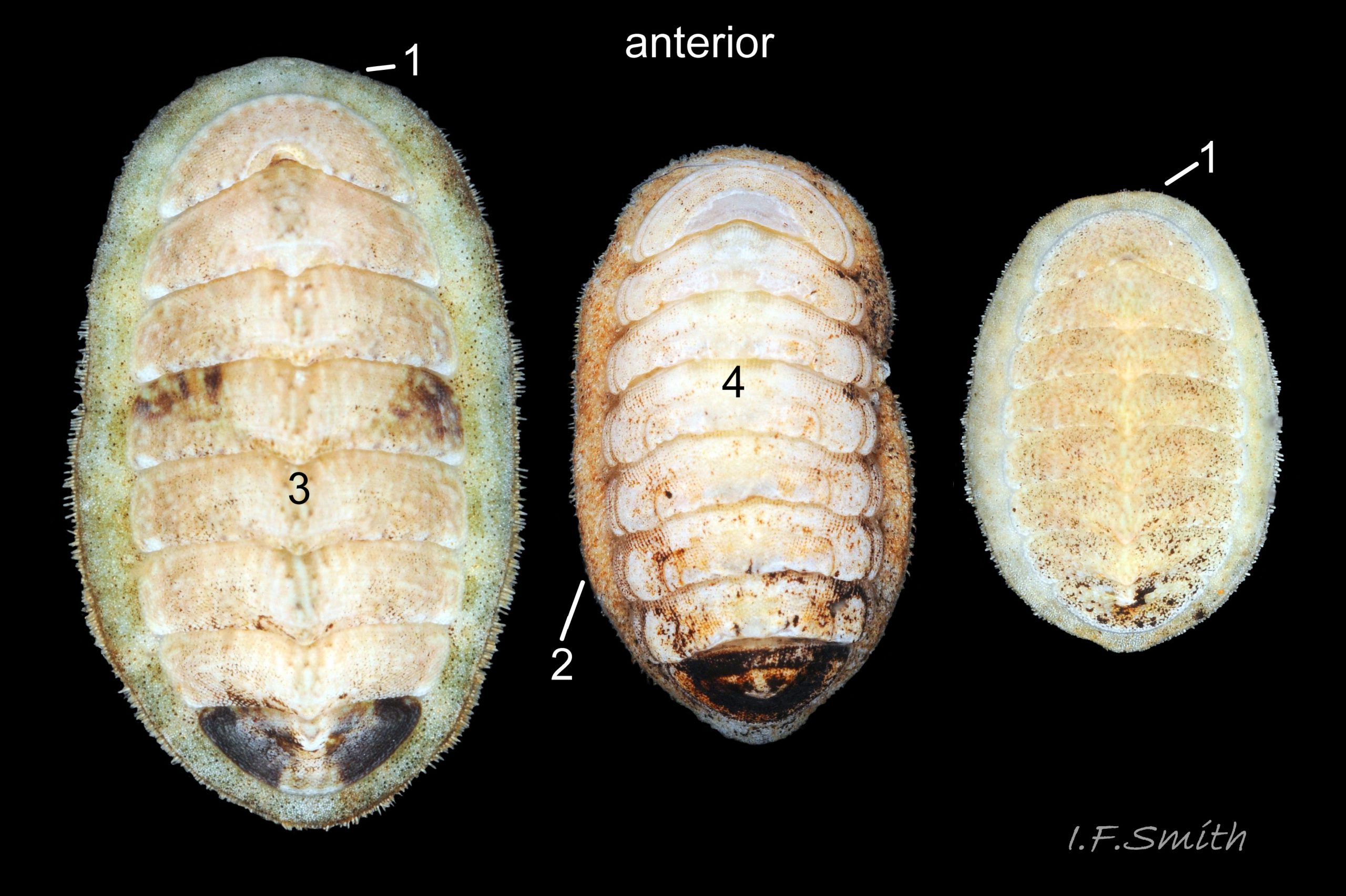
Entire dorsal surface of valves closely shagreened 08 Lepidochitona cinerea by depressed, rounded, slightly rhombic, granules 09 Lepidochitona cinerea that, like seeds in a sunflower, can appear to form curving lines in various directions, especially where least worn near side margins 10 Lepidochitona cinerea. . Granules vary in size and degree of wear 09 Lepidochitona cinerea , and may be unobtrusive on finely-patterned specimens 01 Lepidochitona cinerea . Indistinct growth lines sometimes discernible 03 Lepidochitona cinerea . Jugal area often differentiated by V of different colours 10 Lepidochitona cinerea. , but lateral and pleural areas only sometimes faintly distinguishable by change in orientation of granules on line of insignificant diagonal ridge 11 Lepidochitona cinerea. Colour-containing properiostracum may be thin on this species as unobtrusive and no appreciable immediate loss of dorsal colour after boiling 03 Lepidochitona cinerea , though the canal-riddled tegmentum as a whole decays over long dry-storage of years more than on some other chiton-species. Despite “grey/cinerea” of its names, has great diversity of colours and patterns 23 Lepidochitona cinerea & 24 Lepidochitona cinerea , both dull flic.kr/p/9RAWGe (P.Brazier) and bright 10 Lepidochitona cinerea. . Striking not-infrequent forms have pale dorsal ridge 12 Lepidochitona cinerea. . Some colour forms may resemble those of other species flic.kr/p/c44z1w (P.Lightfoot1) & 25 Lepidochitona cinerea.
Body Description
Head and foot never protrude into view naturally on live animal, can only be seen if animal removed from substrate, or placed on glass.
Head shaped as crescent moon with concave edge fitting round anterior of foot and extending laterally as mouth lappets 13 Lepidochitona cinerea . Much of head occupied by transverse slit-mouth with wrinkled lips 14 Lepidochitona cinerea ; no eyes or sensory tentacles. Pinkish border around head when viewed ventrally 15 Lepidochitona cinerea . As on chitons generally, broad radula with extremely strong hard teeth of chitin mineralized with magnetite in rows of seventeen. Much of radula ribbon is orange. Most teeth colourless or yellow/orange. In each row a pair of major lateral teeth, the principal scrapers, which are pale when formed at rear of radula, darkening to black and hardening with magnetite as moved forwards 16 Lepidochitona cinerea .
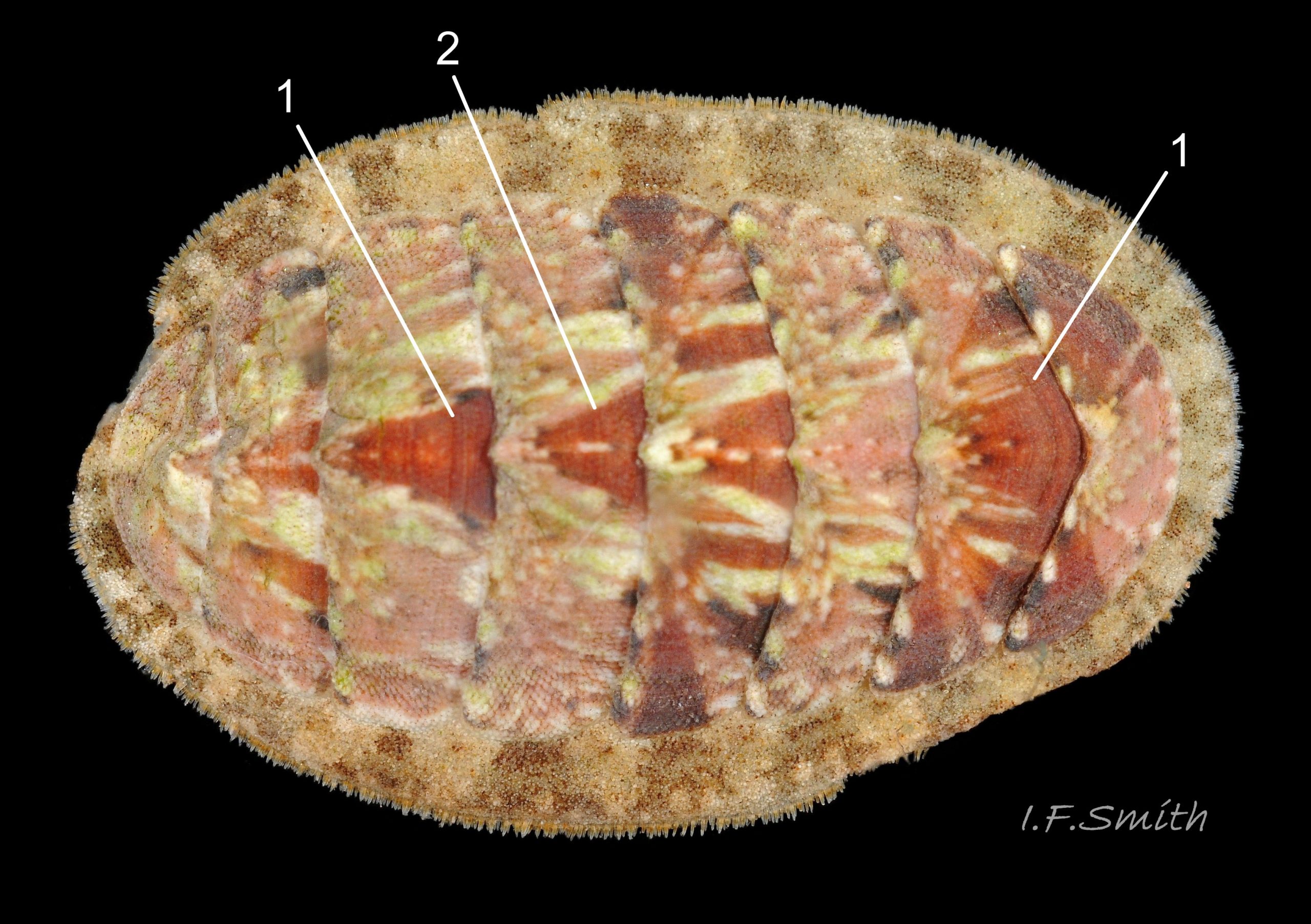
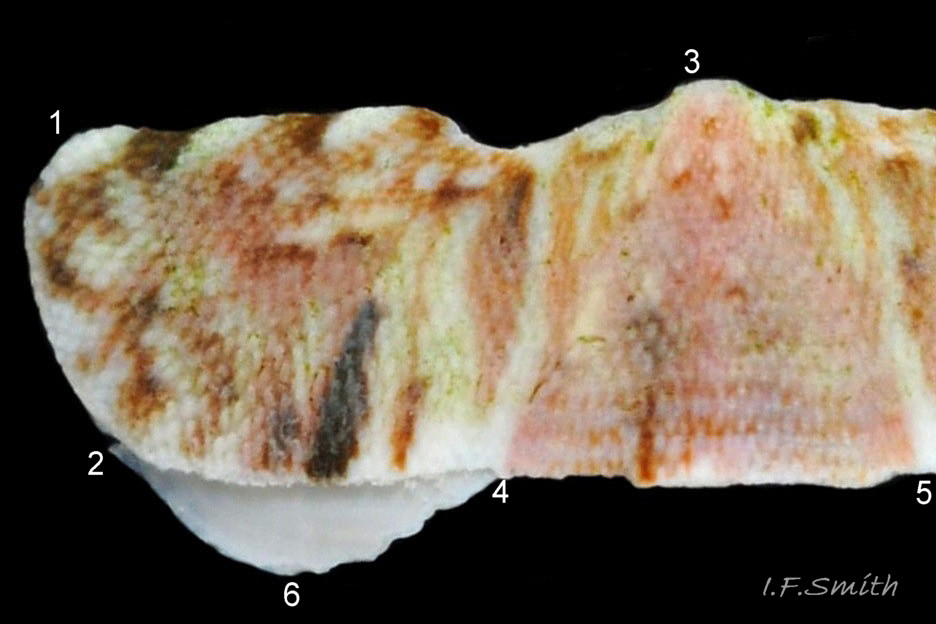
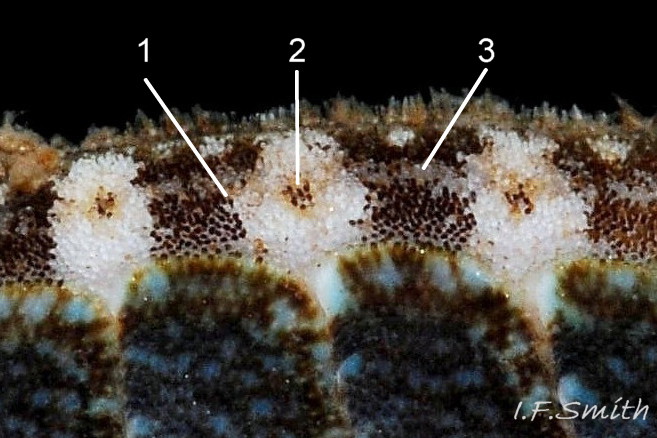
Aesthetes (sensory tissue) fill canals permeating the tegmentum and parts of articulamentum; terminate as sense organs on surface granules 09 Lepidochitona cinerea . Immediately below the valves, mantle is tough thin translucent epidermis, but greatly thickened where reflected around periphery to form a fleshy girdle into which ends of valves deeply embedded 09 Lepidochitona cinerea . Ventral surface of girdle (hyponotum) paved with radiating rows of oblong, scales about 50μm X12μm, often some stained brownish 13 Lepidochitona cinerea . Dorsal surface of girdle (perinotum) has about 90μm-long calcareous corpuscles embedded in it with only the rounded heads exposed as densely-packed rounded granules; usually coloured to form pattern on girdle of alternating dark and light, lozenge-like, transverse bands of approximately equal size. Usually, dark bands have narrow waist, and light bands have bulging waist 17 Lepidochitona cinerea (or vice versa 08 Lepidochitona cinerea ). A usually-thin, pale greyish or pale brownish, longitudinal line often runs across, or close to, waists of bands. Usually a dark central spot on pale bands and sometimes a white spot on dark bands. Markings can be partially developed flic.kr/p/c44z1w (P.Lightfoot1), very indistinct, or absent flic.kr/p/nNRJMS (P.Lightfoot2) on pale specimens. Edge of girdle fringed by translucent golden spines up to 104μm long 15 Lepidochitona cinerea .
Open, narrow mantle-cavity runs around whole animal; contains15 – 19 small ctenidia on each side, extending nearly whole length of foot (holobranch arrangement) 15 Lepidochitona cinerea & 18 Lepidochitona cinerea . Number of ctenidia increases with age. Between mantle-cavity and hypnotum the mantle-fold is unobtrusive except where widens near posterior into small mantle-lappet 15 Lepidochitona cinerea . Anus on papilla opens into mantle-cavity at posterior 14 Lepidochitona cinerea . Excretory nephridopores and gonopores open laterally into posterior quarter of cavity. No penis as external fertilization. Foot, elongate ellipse has pinkish-white 15 Lepidochitona cinerea to orange-pink 14 Lepidochitona cinerea sole with viscera showing as medial band of grey / slate-blue, no medial dividing line. Foot has slightly darker border around sole when viewed ventrally. Foot spreads widely, concealing mantle-cavity when gripping substrate 13 Lepidochitona cinerea .
Key identification features
Lepidochitona cinerea (Linnaeus, 1767).
a) Only chiton species likely to be found higher than MLW on British shores. Has diverse colour forms , some similar to other spp. such asTonicella rubra or Leptochiton cancellatus.
b)Dorsal surface of valves has numerous rounded, sometimes slightly elongated, granules visible at X5 magnification 09 Lepidochitona cinerea .
c) Apophyses on intermediate valves wide, rounded, slightly-triangular.
d)Dorsal surface of girdle has densely packed rounded granules 09 Lepidochitona cinerea .
e)Girdle usually has alternating dark and light transverse lozenge-like bands of approximately equal size. Dark bands usually have narrow waist, and light bands usually have a bulging waist 17 Lepidochitona cinerea (or vice versa 08 Lepidochitona cinerea ). A thin paler longitudinal line often runs across the waist of dark bands. Usually a dark central spot on pale bands. Markings can be partially developed, very indistinct, or absent flic.kr/p/nNRJMS (P.Lightfoot2) on pale specimens.
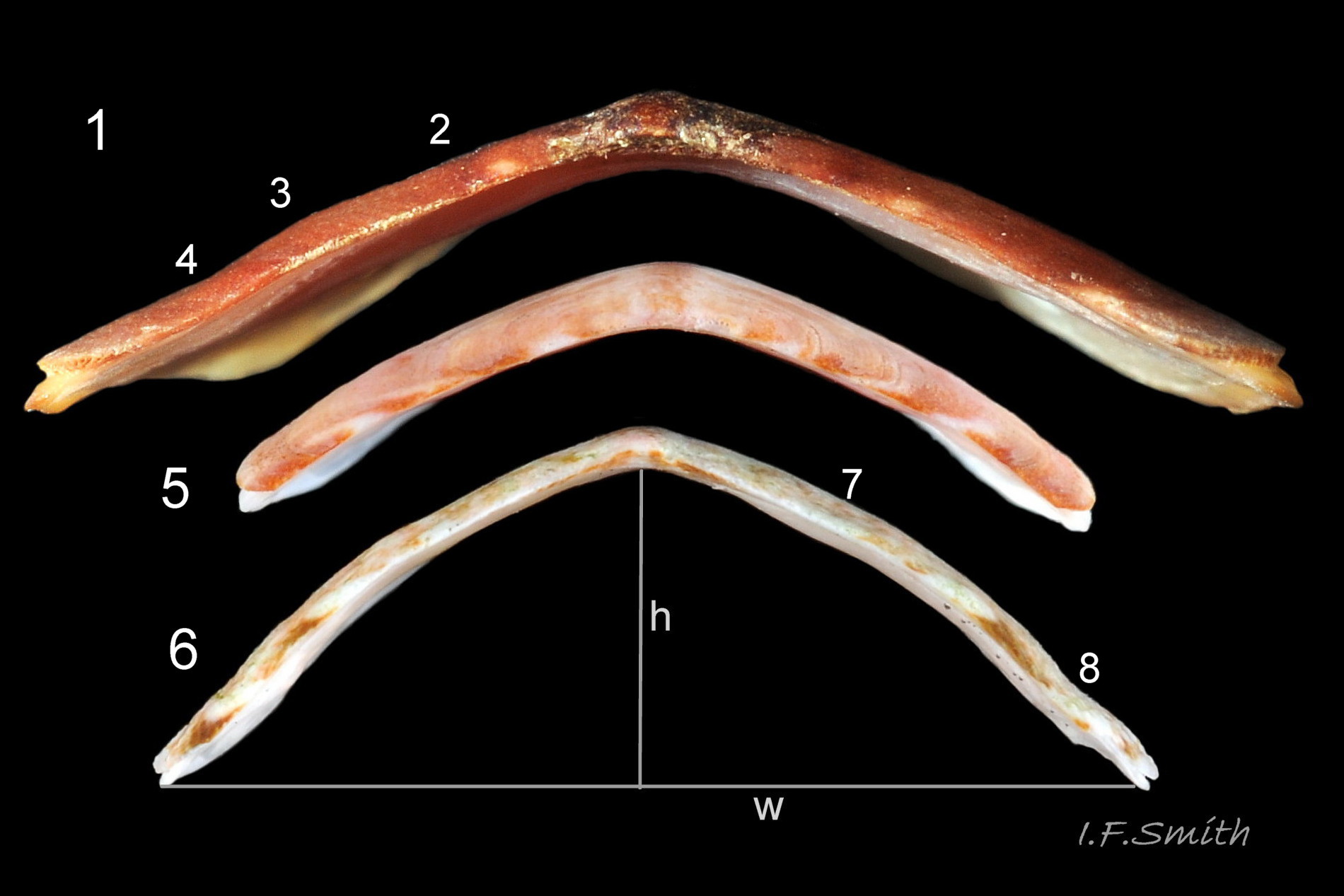
f) Intermediate valves moderately highly arched with keel and posterior beak; valve 5 height about 32% of width; distinctly higher than that of Tonicella rubra 05 Lepidochitona cinerea.
g)Usually 16 to19 ctenidia each side, arrangement holobranch 15 Lepidochitona cinerea .
h) All round British isles on rocky shores.
Similar species
Leptochiton cancellatus 26 Lepidochitona cinerea
a) At LWS and sublittorally.
b) Dorsal surface of valves has many closely packed granules in almost-straight chain-like rows running antero-posteriorly and, usually, a few strong growth lines.
c) Narrow triangular apophyses on intermediate valves.
d) Dorsal surface of girdle has densely-packed squarish scales with curled up tips.
e) No distinctive pattern on girdle.
f) Intermediate valves highly and smoothly arched with neither keel nor posterior beak 25 Lepidochitona cinerea & 26 Lepidochitona cinerea .
g) About five ctenidia on each side near posterior; merobranch arrangement.
h) Widespread around Britain, scarce in southern North Sea.
Tonicella marmorea O. Fabricius, 1780.
a) At LWS and sublittorally.
b) Dorsal surface of valves has growth lines and, at X5 magnification, numerous distinct small granules 17 Boreochiton ruber. (Granules, not as large as on L. cinerea, may not be discernible in underwater images or on specimens before deposits and properiostracum removed.)
c) Apophyses on intermediate valves short (i.e. anterior-posterior), wide and gently curved 17 Boreochiton ruber. (Note, apophyses in 4 in Kaas & Belle appear to be worn/damaged to stronger curve. Fig.19 in Jones & Baxter shows un-damaged apophyses more accurately.)
d) Dorsal surface of girdle has sparse minute granules(c.27μm long) that do not touch each other. (Usually escape notice in photographs.)
e) Girdle variably coloured and patterned; deep purple-red to green and may have orange bands; some may resemble T. rubra.
f) Dorsal surface of valves ii to vii flat or weakly convex upper slope, separated by hip from slightly concave lower slope 05 Lepidochitona cinerea. Low keeled arch, similar height/width ratio to T. rubra.
g) 17 to 26 ctenidia each side, arrangement merobranch.
h)Not found south of North Wales or Northumberland. (Many erroneous records on mapping schemes as easily confused with T. rubra.)
Tonicella rubra (Linnaeus, 1767).
a) At LWS and sublittorally.
b)Dorsal surface of valves smooth apart from growth lines. (Faint stippling may be visible at X30 magnification.) 17 Boreochiton rube.
c)Apophyses, especially on valves iii to v, are markedly prominent and rounded, almost semicircular 17 Boreochiton ruber.
d) Dorsal surface of girdle has densely-packed, elongate (50 – 60μm), club-shaped granules with packed touching heads giving a mealy appearance. 14 Boreochiton ruber .
e)Girdle usually has alternating dark and pale transverse bands. Pale bands, often fragmentary, usually thinner than dark bands, and roughly in line with shell-sutures 02 Boreochiton ruber .
f)Dorsal surface of valves ii to vii makes a keeled arch with smoothly-curved or flattish sides when viewed in profile from posterior 05 Lepidochitona cinerea. Keeled arch distinctly lower than that of Lepidochitona cinerea, but only slightly higher than on T. marmorea.
g) About 12 ctenidia each side (range 10 to15), arrangement merobranch 12 Boreochiton ruber .
h) Widespread around Britain except southern North Sea.
Habits and ecology
Commonest littoral species of chiton in Britain; from middle shore to Laminaria zone, and sublittorally; to 70m in N. Norway. When tide is out, lives under stones, especially ones lightly embedded in sand or gravel in rock pools. When immersed, positively phototactic and negatively geotactic so moves up into well lit areas where algal food prospers. When emersed, taxis reversed so moves down into shade and moisture to avoid desiccation. Shell articulates to conform closely to uneven rock surface 20.1 Lepidochitona cinerea ; large foot and flexible girdle grip surface firmly. When alarmed, can increase grip suctorially by raising part of girdle to form partial vacuum, and if displaced from substrate, can roll into a ball 20 Lepidochitona cinerea & 22 Lepidochitona cinerea .
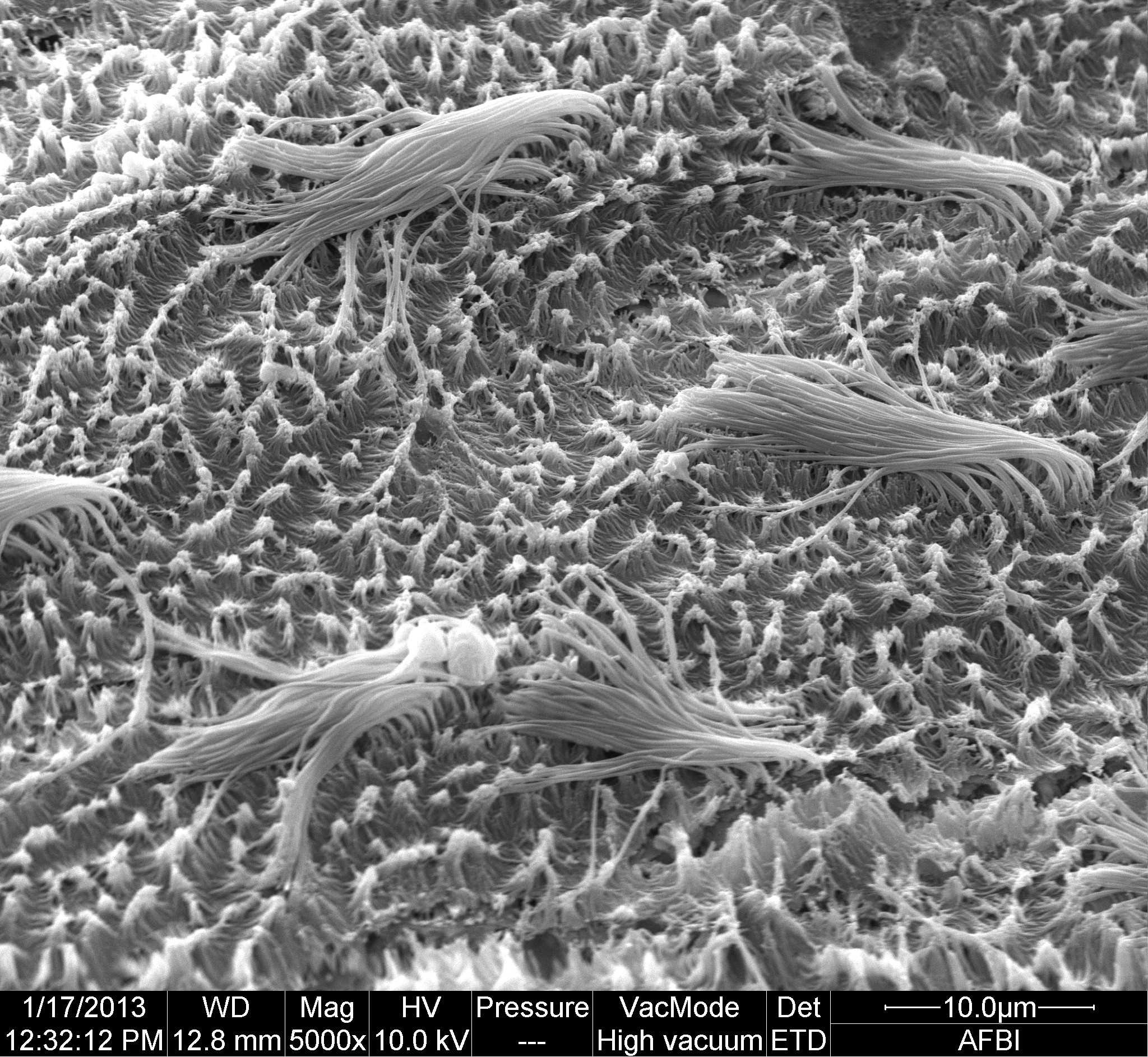
Respiration: tufts of cilia, 15 to 20 μm long, on surfaces of mantle-cavity and ctenidia 18 Lepidochitona cinerea create inhalent water-current entering inhalent chamber of mantle-cavity wherever girdle is raised laterally. Adjacent ctenidia have interlocking cilia so all ctenidia work as a unit (as in many bivalves).
Current passes through ctenidia into exhalent chamber and along it as exhalent current to posterior to exit under raised girdle 19 Lepidochitona cinerea . Surface between cilia tufts is carpeted with smaller microvilli; precise function uncertain.
Circulation: blood travels from head sinus through longitudinal sinuses to the foot, viscera and mantle, then through the ctenidia 15 Lepidochitona cinerea for aeration before passing into the adjacent heart in the dorso-ventrally flattened pericardium below valves vii & viii. From the heart the blood is pumped through the medial dorsal aorta to the head sinus, giving off some channels to the gonad and valve muscles on the way.
In absence of eyes, or of tactile or chemo-receptor tentacles on head, senses environment through aesthetes on surface of shell. Precise sensory functions of aesthetes uncertain, but focusing lens and receptive retina found in aesthetes of some chiton species. Other proposed aesthete functions include chemoreception, mechanoreception, replenishment of properiostracum materials, and secretion of protective substances. Also sensory organs on girdle.
Feeds by scraping microalgae and associated organisms from the rock surface using its hard radula of chitin mineralized with magnetite 16 Lepidochitona cinerea . Though primitive in several ways, has elaborate digestive system with no trace of primitive rotating style in stomach. Long coiled intestine compacts faeces into oval pellets. Water current in mantle-cavity carries excreta from lateral nephridopores to posterior, where faecal pellets from anus join the flow; all expelled at posterior.
Trichodina sp. ciliate protozoan parasites, 30 μm wide, sometimes heavily infest the mantle-cavity. Travels by monotaxic retrograde compression waves on sole of foot 14 Lepidochitona cinerea . Breeding: dioecious. Water current in mantle-cavity carries sperm or ova from lateral gonopores to exit at posterior. As fertilization is external, synchronised emission of sperm and ova needed to ensure success; trigger in many chiton species is moon-phase/ state of tides. Female lays adhesive gelatinous ribbon of spawn containing greenish ova 21 Lepidochitona cinerea . Planktonic trochophore larvae hatch and metamorphose into small adult-form young without intervening veliger stage.
Distribution and status
Widespread, and common on many rocky shores. North Norway to Mediterranean and Black Sea. Not Iceland or inner Baltic. GBIF map www.gbif.org/species/2306437 . All around Britain and Ireland, but scarce in Liverpool Bay and Flamborough to Ipswich. NBN U.K. map species.nbnatlas.org/species/NHMSYS0021056550
Acknowledgements
Grateful thanks are due to Paula Lightfoot for providing specimens and images, and to Paul Brazier, Daniel Lombard, Neil Roberts and Mike Rutherford for use of their images. Special thanks to Dr Lauren Sumner-Rooney of the Marine Laboratory, Queens University, Belfast, for use of a scanning electron microscope image of pallial cilia. I am indebted to Dr Julia Sigwart, Director of the Marine Laboratory, Queens University, Belfast, for detailed advice with the text and interpretation of the images. Any errors or omissions are the responsibility of the author.
Links and references
Botelho, A. 2013. Zoologger: mollusc grows hardest teeth in the world New Scientist 3rd October 2013 www.newscientist.com/article/dn24329-zoologger-mollusc-gr…
Forbes, E. & Hanley S. 1849-53. A history of the British mollusca and their shells. vol. 2 (1853) London, van Voorst. (As Chiton cinereus; Free pdf at archive.org/details/historyofbritish02forbe Use slide at base of page to select pp. 402 – 405.)
Fox, R. 2007. Invertebrate Anatomy On Line. Katharina tunicata lanwebs.lander.edu/faculty/rsfox/invertebrates/katharina….
Jardim, J.A. & Simone, L.R.L. 2010. Redescription of Hanleya brachyplax (Polyplacophora, Hanleyidae) from the south-southeastern Brazilian coast. Pap. Avulsos Zool. (São Paulo) 50 no.40
www.scielo.br/scielo.php?pid=S0031-10492010004000001&…
Jeffreys, J.G. 1862-69. British conchology. vol. 3 (1865). London, van Voorst. (As Chiton marginatus; Free pdf at archive.org/details/britishconcholog03jeffr . Use slide at base of page to select pp.221 – 224.)
Jones, A.M. & Baxter, J.M. 1987. Molluscs: Caudofoveata, Solenogastres, Polyplacophora and Scaphopoda London, Linnean Society, and Estuarine and Brackish-water Sciences Association.
Kaas, P. & Van Belle, R.A. 1985. Monograph of living chitons Vol 1. Viderup, Denmark. Brill. Preview at
books.google.co.uk/books?id=CKautf2EUPkC&pg=PA17&…
Matthews, G. circa1954. The identification of British chitons. Papers for Students No.9. London, Conchological Society of Great Britain and Ireland.
Montagu, G. 1803. Testacea Britannica or natural history of British shells, marine, land, and fresh-water, including the most minute: Systematically arranged and embellished with figures. London, J. White. [p. 3] www.biodiversitylibrary.org/item/78694#page/53/mode/1up
Schwabe, E. 2010. Illustrated summary of chiton terminology. Spixiana 33(2):171-194.
Free pdf at verlag-pfeil.de/04biol/pdf/spix33_2_02.pdf
Sumner-Rooney, L.H., Cain,S., Brennan, G.P. & Sigwart, J.D. 2014. A test for mucus removal in the chiton Lepidochitona cinerea (Linnaeus, 1776) (Polyplacophora: Chitonida: Ischnochitonidae). The Veliger 51(4): 261-264. www.academia.edu/5796930/A_test_for_mucus_removal_in_the_…
Glossary
μm – 0.001 mm
aesthetes – complex of canals and cavities filled with sensory tissue that permeate tegmentum and locally penetrate articulamentum. Open as sensory pores on dorsal surface of valves; probably compensate for lack of sensory structures on head. Some sense light; other sensory function(s) uncertain, but various authors have variously proposed chemoreception, mechanoreception, properiostracum replenishment and secretion of protective substances.
afferent – (adj. of vessel) carrying blood etc towards an organ.
a.k.a. – also known as.
antemedian – (syn. antemedial) situated to anterior of middle.
antemucronal area – area situated to anterior of mucro.
apophysis – (pl. apophyses) natural protruberance within a shell for attachment of muscles. On chitons, (a.k.a. sutural lamina) anterior extension of articulamentum which underlies preceding valve; on all valves except head valve (i).
aragonite – orthorhombic crystalline mineral-form of calcium carbonate www.minerals.net/mineral/aragonite.aspx . Less common on land than calcite, but, currently, the more frequent mineral-form in oceans and living mollusc shells.
articulamentum – inner shell-layer of chiton valves, usually hard, white, porcelaneous aragonite and often differently coloured in central part. (Partially underlain by inconspicuous myostracum layer.)
buccal mass – anterior of digestive system including an odontophore that supports anterior of radula, and a complex of muscles to operate them and other mouthparts. Usually red or pink.
cephalic – (adj.) of or on the head.
chemoreception – sensing of chemicals; “smell / taste”.
chitin – semitransparent flexible horny protein.
chitinous – (adj.) made of chitin.
cilia (pl.) –motile linear extensions of membrane used in locomotion, or to create water currents in feeding. (“cilium” singular).
coll. – in the collection of (named person or institution) (cf. legit).
ctenidium – comb-like molluscan gill; usually an axis with a row of filaments either side.
dioecious – having separate male and female individuals, not hermaphrodite.
dorso-ventrally flattened – as if pressed flat from above.
efferent – (adj. of vessel) carrying blood etc away from an organ.
emersed – above water surface, not covered in water.
epithelium – tissue forming outer layer of body surface, “skin”.
geotactic – (adj.) of species that moves towards pull of gravity (positively geotactic) or away from it (negatively geotactic). (synonym: gravitaxic.)
girdle – (on chiton) peripheral band of thickened, reflexed mantle that encloses ends of valves.
gonopore – genital opening through which eggs or sperm are released.
holobranch – (of chitons) ctenidia in mantle-cavity extend full length of foot.
immersed – covered in water.
insertion plate – (on most chitons) extension of articulamentum on lateral margin of intermediate valves, anterior margin of head valve and posterior margin of tail valve. Inserts into, and anchors valve to, the girdle muscle block.
intermediate valve – (of chiton) any valve (ii – vii), except head valve (i) and tail valve (viii).
interspecific – (adj.) existing or arising between different species.
intraspecific – (adj.) occurring within a single species or involving members of one species.
jugal area – triangular middle section of central area of intermediate valves, with apex pointing to posterior; discernible when defined by differences of colour and/or sculpture (dorsal surface).
jugal tract – triangular middle section of central area of intermediate valves, with apex pointing to posterior; discernible when defined by densely arranged aesthete pores (ventral surface).
jugum – triangular middle section of central area of intermediate valves. (See jugal area and jugal tract.)
lateral area – (on intermediate valve of chiton) triangular area with its base along lateral edge of valve and its apex near the centre of the posterior edge. a.k.a. lateral triangle.
legit – (abbreviation; leg. or lgt.) collected/ found by (cf. coll.)
LWS – low water spring tide, two periods of a few days each month when tide falls lowest.
magnetite – mineral of iron oxide, hardest material made by any living organism (Botelho, 2013).
major lateral teeth– largest two teeth in each row of seventeen on chiton radulae; principal scraping blades. Often darkened more than other teeth by high magnetite mineralization.
mantle – sheet of tissue that secretes the shell, surrounds the viscera and forms a cavity for the gill in most marine molluscs.
mantle-cavity (on chitons)= narrow groove around whole foot and head, roofed by mantle and containing ctenidia, nephridiopores and gonopores. a.k.a. pallial groove.
mechanoreception – sensing of touch, sound, pressure change and/or posture.
microvilli (pl.) – microscopic cellular membrane protrusions that increase the surface area of cells. Possible functions include absorption, secretion, cellular adhesion and mechanotransduction. (singular, microvillus).
MLW – mean low water mark.
monotaxic – (of locomotion waves on foot) single series of waves; each wave extends across complete width of foot.
merobranch – (of chitons) gills in pallial cavity only in posterior two-thirds of animal.
motile – (adj.) able to move spontaneously; applicable to whole animals or to parts of them such as cells, gametes or cilia.
mucro – projection on tail valve (viii) of chiton demarking posterior from rest of valve. Varies in prominence and position.
myostracum – thin, inconspicuous, discontinuous, innermost layer of chiton shell.
nephridium – tubular glandular excretory/ osmoregulatory organ. a.k.a. kidney.
nephridiopore –opening of nephridium for excretion. a.k.a. nephropore, or renal pore.
odontophore – firm, approximately ellipsoid, structure of cartilage supporting radula. Protruded like a tongue to operate radula.
osmoregulation – regulation of osmotic pressure to keep organism’s fluids from becoming too diluted or concentrated.
pericardium – membranous sac containing heart and start of aorta.
perinotum – dorsal surface of chiton’s girdle.
pleural area – (on intermediate valve of chiton) triangular area with its base along anterior edge of valve and its apex near the centre of the posterior edge. a.k.a. median triangle.
postmucronal – situated to posterior of mucro on tail valve.
properiostracum – (on chitons) proteinaceous material covering the shell. Different composition from periostracum of most other molluscs.
phototactic – (adj.) of species that moves towards light (positively phototactic) or away from it (negatively phototactic).
plankton – animals and plants that drift in pelagic zone (main body of water).
radula – ribbon of chitin bearing chitinous teeth that is extruded on a tongue-like odontophore of cartilage to rasp food. On chitons, teeth are usually impregnated with magnetite, a hard magnetic mineral of iron.
rec. – recorded by (person who submitted record, may be different from leg. and coll. persons/institution).
retrograde – (of locomotion waves on foot) waves travel from anterior to posterior.
side slope – (on chiton) shape in profile view (from posterior or anterior) of lateral areas of intermediate valves; may be straight, convex, concave or a combination of these.
sinus – dilated channel or receptacle containing blood etc.
slit ray -– row of canal pores running diagonally from lateral slit to posterior edge on ventral surface of chiton valve. a.k.a. notch ray.
suture – (of chiton) line where two valves meet.
taxis – directional locomotary response to external stimuli such as light, gravity, temperature or chemicals.
tegmentum – outer shell-layer of chiton valves, usually porous and relatively soft. (Covered by properiostracum when live.)
trochophore – spherical or pear-shaped larvae that move with aid of girdle of cilia. Stage preceding veliger, passed within gastropod egg in most spp. but free in plankton for limpets, Trochidae, Tricolia pullus and (with no veligers) chitons.
veliger – shelled larva of marine gastropod or bivalve mollusc which swims by beating cilia of a velum (bilobed flap).